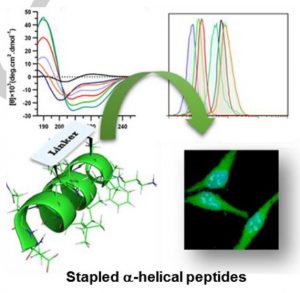
Stapled peptides emerged as a new class of targeting molecules of high binding affinity and specificity for intracellular undruggable targets. Their ability to penetrate cell membranes were exceptionally intriguing to the field, yet elusively and controversially discussed. To understand the effect of stapling architectures on their physiochemical properties and to aid in promoting their cell permeability, we report herein a comparative study on physiochemical properties and cell permeability of stapled α-helical peptides with different types of cross-links. We highlight the decisive impact of intrinsic properties of the cross-links on cell permeability rather than peptides’ helical contents in a model amphipathic sequences targeting estrogen receptor – coactivator interaction. We envision this finding to further shed light on chemical optimization of stapled α-helical peptides or macrocyclic cell penetrating peptides for enhanced cell penetration.
Link:http://onlinelibrary.wiley.com/doi/10.1002/cbic.201700352/abstract