2014 first JACS, Congratulations to Li-Ping
吴云东教授课题组在新型不对称Heck反应机理研究上取得重要进展
Heck反应因其温和的反应条件,高效的合成手段几十年来一直被广泛应用于碳碳键偶联反应中。不对称的Heck反应可以产生不对称的手性中心,在天然产物合成及药物合成中吸引了人们的关注。然而,现在大多数的不对称Heck反应仍然很难获得高的选择性,一般都需要有诱导基团的底物或者环状的底物来达到选择性的控制。Sigman教授最近报道了一类具有高的区域选择性和对映选择性的不对称Heck反应。在这个新型反应中,他们使用了非环状的烯基醇底物,得到了高对映选择性的不对称芳基醛/酮产物,区域选择性倾向于芳基连在离羟基较远的烯基碳上,不饱和性通过若干步迁移插入和beta-H消除最终传递在羟基上。
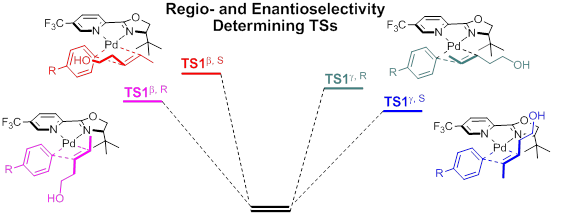
为了探明决定此反应区域选择性和对映选择性的因素,以及手性配体对于不饱和性传递效率的影响,吴云东教授课题组对这类新型的不对称Heck反应进行了理论研究。理论计算结果表明,区域选择性是由远端羟基的C-O偶极诱导产生的电子效应决定的,而对映选择性则是由配体与底物之间的空间位阻效应所决定的。相对平缓的势能面也反映出不饱和性传递的高效性。最后,根据理论计算做出的不同底物反应性和选择性的预测也被Sigamn教授课题组进行的实验所证实。通过理论计算与实验验证,我们对这类新型不对称Heck反应的机理有了深入而且全面的认识,为该反应的改进和新反应的发现提供了启示。该文章目前以全文形式发表在近期的《美国化学会志》上(J. Am. Chem. Soc., DOI: 10.1021/ja4109616)。
我院研究生徐立平同学负责该工作理论计算研究,为该文章第一作者。
文章链接:http://pubs.acs.org/doi/abs/10.1021/ja4109616
Yun-Dong Wu’s Group Made Progress in Novel Asymmetric Heck Reaction Mechanism
Heck reaction is an excellent method to introduce an aromatic group to one end of a double bond using palladium(0) catalysis. The asymmetric variant of the Heck reaction has received great attention as it generates chiral centers when forming new carbon-carbon bonds, of vast importance in natural product and pharmaceutical syntheses. Among most of the asymmetric Heck reactions, however, there are still limitations of achieving high site- and enantioselectivity. Recently, Sigman’s group reported a highly regioselective and enantioselective Heck reactions of electronically nonbiased alkenes without chelating groups. This type of reaction is very attractive for two reasons: first is the high site selectivity and enantioselectivity with unbiased achiral substrates; and second is the simultaneously functionalization of multiple carbons through a presumably iterative b-hydride elimination/migratory insertion process.
To understand the effects that control the site- and enantioselectivity, and the ligand effect on the relay strategy, Professor Wu’s group conducted detailed computational studies on this Pd-catalyzed redox-relay Heck arylations of alkenyl alcohols. The calculations showed that the regiochemistry is controlled by a remote electronic effect, where the developing polarization of the alkene in the migratory insertion transition state is stabilized by the C–O dipole of the alcohol moiety. The enantioselectivity, on the other hand, is controlled by steric repulsion between the oxazoline substituent and the alcohol-bearing alkene substituent. The relay efficiency is due to an unusually smooth potential energy surface without high barriers, where the hydroxyalkyl-palladium species acts as a thermodynamic sink, driving the reaction towards the carbonyl product. At last, the computational predictions of the relative reactivity and selectivity are valided experimentally. These results provide detailed mechanistic insights into this novel reaction and will allow further improvements for cases where the selectivity of the reaction needs to be enhanced. This work has been published recently at J. Am. Chem. Soc. (DOI: 10.1021/ja4109616).
This article can be accessed at: http://pubs.acs.org/doi/abs/10.1021/ja4109616