Wu group published article on Chem. Sci. about Diversity-Oriented Synthesis of Bioactive Benzanilides by a Regioselective C(sp2)-H Hydroxylation Strategy
Hydroxylated benzanilides are an important class of chemicals used frequently in pharmaceuticals and agrochemicals. The position of hydroxyl group, i.e. at the ortho-position in either the benzoyl or the aniline moiety, may underlie dramatically different bio-activities. For example, in a DNA-PK inhibitor the hydroxyl group is on the benzoyl segment, but it is on the aniline ring in a rotamase inhibitor. Different regioselectivity was observed with Ru(II) and Pd(II) catalysts. The reaction demonstrates excellent regioselectivity, good tolerance of functional groups, and high yields. A wide range of ortho-hydroxylated-benzanilides can be readily synthesized with excellent regioselectivity by this new synthetic strategy.
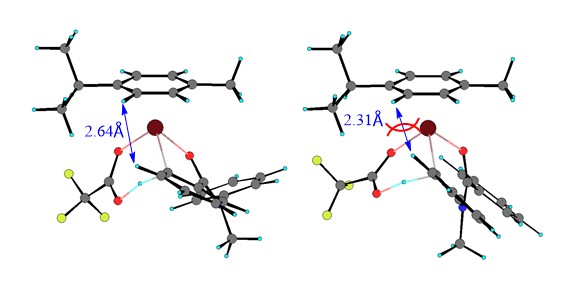
Computational investigations revealed that the regioselectivity was controlled mainly by both steric and electronic factors. Steric effects determine the regioselective outcomes in the Ru-catalyzed reaction, while electronic effect are dominant in the Pd-catalyzed reaction. This highlights the importance of considering the coordination geometries preferred by a metal center when performing carboxylate-assisted transition-metal catalyzed C-H activation reactions.
Ph.D. student Tian-Yu Sun is the co-first author, Professor Xinhao Zhang is the co-corresponding author of this paper. This article was published in the "Chemical Sciences" (rao, y .; Wu, Y.-D .; Zhang, X .; sun, y .; sun, t. Chemical Science 2015. A Diversity-Oriented Synthesis of Bioactive Benzanilides by a Regioselective C (sp2) -H Hydroxylation Strategy DOI:. 10.1039 / c5sc03905c)