122.Total Synthesis of Sinensilactam A
Wenbin Shao, Jun Huang, Kai Guo, Jianxian Gong*, and Zhen Yang*
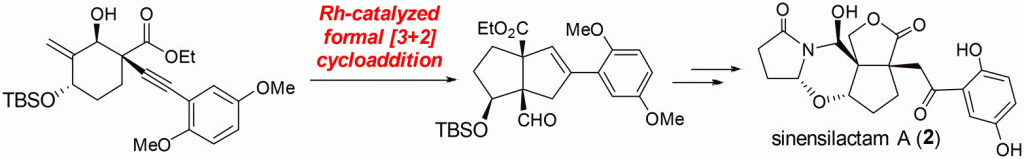
The total synthesis of naturally occurring (±)-sinensilactam A was achieved in 18 steps. The key steps of this work are a rhodium-catalyzed [3 + 2] cycloaddition for construction of the two all-carbon vicinal quaternary centers and a convergent and tandem condensation of the in situ generated N-acyliminium intermediate with aldehyde 20. This enabled implementation of a unified strategy for stereoselective formation of the tetracyclic hemiaminal core of sinensilactam A in a later stage. The total syntheses of applanatumol F and C8-epi-applanatumol D are also achieved using this strategy.