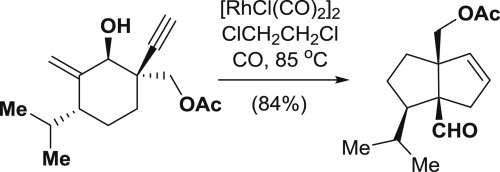
135.Concise synthesis of the core structure of madreporanone by Rh-catalyzed [3+2] cycloaddition
Rong Long, Zhen Yang*
Tetrahedron 2019, 75 (12), 1746-1750
A model study toward total synthesis of madreporanone, a novel diterpene isolated from Azorella madreporica, was investigated. The [3.3.0] bicyclic core of madreporanone bearing a cis-configured isopropyl group and two vicinal quaternary carbon centers was stereoselectively constructed via an intramolecular Rh-catalyzed [3 + 2] cycloaddition in a single step.