138.Asymmetric Total Synthesis of Pre-schisanartanin C
Yan-Long Jiang, Hai-Xin Yu, Yong Li, Pei Qu, Yi-Xin Han, Jia-Hua Chen*, Zhen Yang*
J. Am. Chem. Soc.2020, 142(1), 573-580
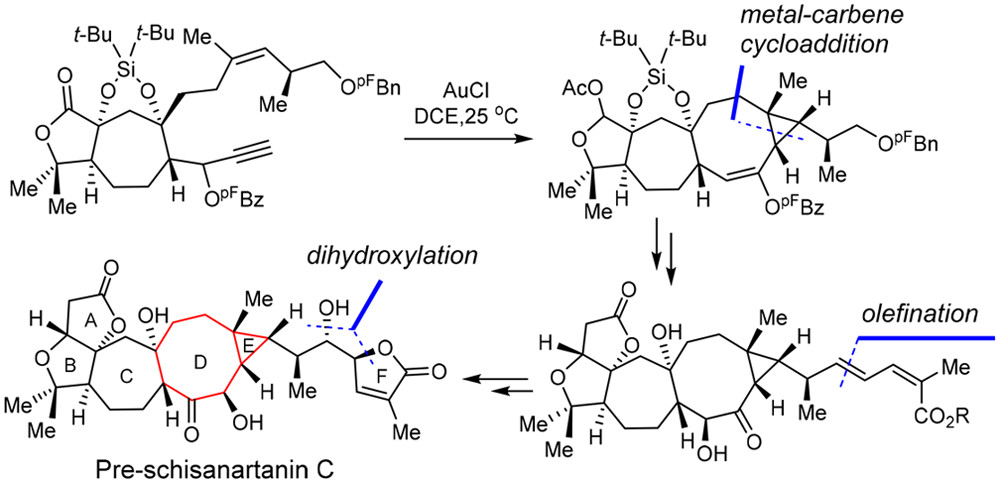
Pre-schisanartanin C belongs to the family of Schisandra nortriterpenoids with potent antihepatitis, antitumor, and anti-HIV activities. This paper presents the enantioselective total synthesis of pre-schisanartanin C (1). An important step in the total synthesis of 1 is gold-catalyzed intramolecular cyclopropanation of an 1,8-enyne substrates bearing a secondary ester group at the propargylic position to prepare a bicyclo[6.1.0]nonane core. Additional highlights include i) an asymmetric Diels–Alder reaction to install the initial C5 stereogenic center of 1, and ii) a sequential Pd-catalyzed Stille coupling, regio- and stereo-selective Sharpless asymmetric dihydroxylation, and a subsequent intramolecular lactonization to contruct the side chain of 1. The developed chemistry paves the way for the total syntheses of other family members bearing highly rigid bicyclo[6.1.0]nonane cores.