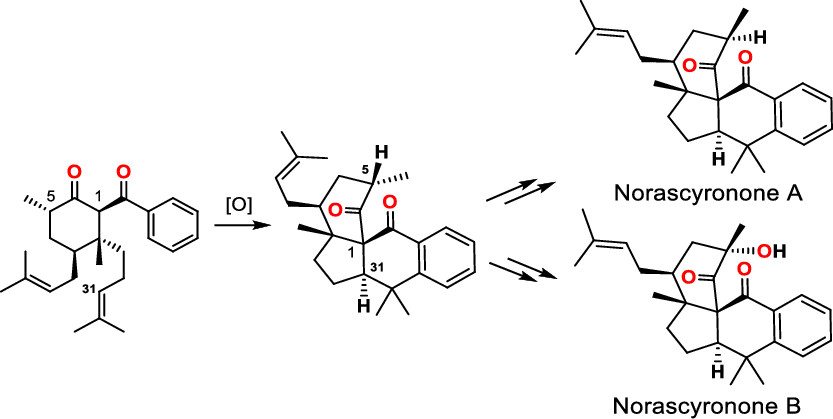
141.Protecting-Group-Free Total Syntheses of (±)-Norascyronones A and B
Tingting Cao, Lei Zhu, Yu Lan*, Jun Huang* and Zhen Yang*
Org. Lett. 2020, 22(7), 2517-2521
Protecting-group-free total syntheses of natural products norascyronone A and norascyronone B were accomplished in eight steps from the commercially available starting material 1-bromo-4-methoxy-2-methylbenzene. The key step was a Mn/Cu-mediated oxidative cascade annulation reaction that formed the tetracyclic core of the target molecules bearing vicinal bridge-head all-carbon quaternary chiral centers. Our investigation indicated that the C5 stereogenic center of norascyronone C plays a critical role in the proposed biomimetic oxidative reaction for B-ring formation.