145.Asymmetric Total Synthesis of (−)-Spirochensilide A
Xin-Ting Liang, Jia-Hua Chen,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(18), 8116–8121
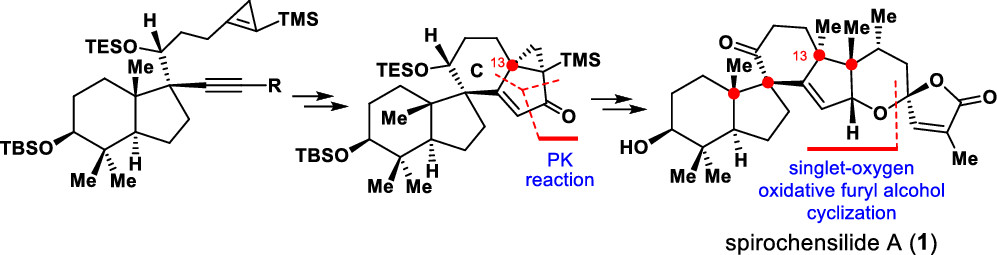
An asymmetric total synthesis of (−)-spirochensilide A has been achieved for the first time. The synthesis features a semipinacol rearrangement reaction to stereoselectively construct the two-vicinal quaternary chiral centers at C8 and C10, a tungsten-mediated cyclopropene-based Pauson–Khand reaction to install the C13 quaternary chiral center, and a furan-based oxidative cyclization to stereoselectively form the spiroketal motif.