151.Asymmetric Total Synthesis of (−)-Spirochensilide A, Part 2: The Final Phase and Completion
Xin-Ting Liang, Bao-Chuan Sun, Nan Zhang, Zhong-Chao Zhang, Yuan-He Li, Qian-Qian Xu, Chang Liu, Jia-Hua Chen,* and Zhen Yang*
J. Org. Chem. 2021, 86(3), 2158–2172
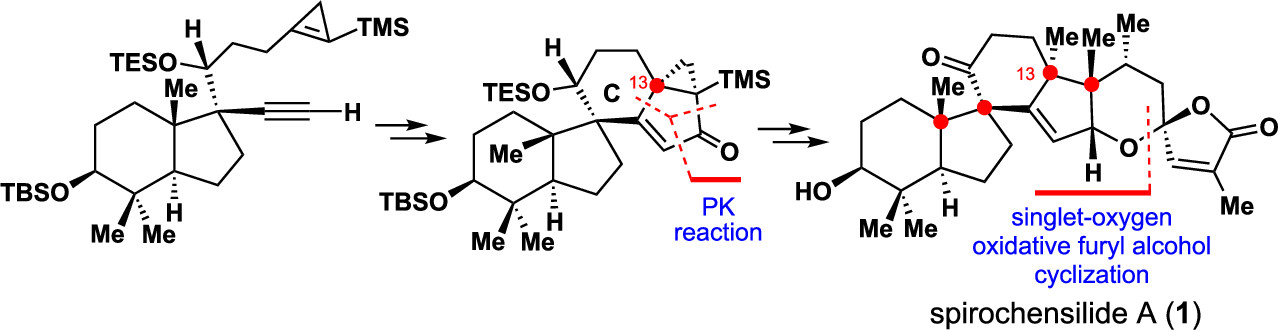
The final phase of the total synthesis of (−)-spirochensilide A is described. A tungsten-mediated cyclopropene-based Pauson–Khand reaction was developed to form the spiral CD ring system with desired stereochemistry at the C13 quaternary center. Other important steps enabling completion of this synthesis included an intermolecular aldol condensation to link the ABCD core with the EF fragment and a Cu-mediated 1,4-addition to stereoselectively install the C21 stereogenic center. The chemistry developed for this total synthesis of (−)-spirochensilide A (1) will aid the synthesis of polycyclic natural products bearing this unique spiral ring system.