169.Regioselective Hydroxylation of Flavonoids by Transition-Metal-Catalyzed C−H Bond Oxidation
Shu-Min Lu, Chao Chen, Chang Liu, Rudong Liu, Jia-Hua Chen,* Zhongchao Zhang,* and Zhen Yang*
Org. Lett. 2023, 25(13), 2264–2269
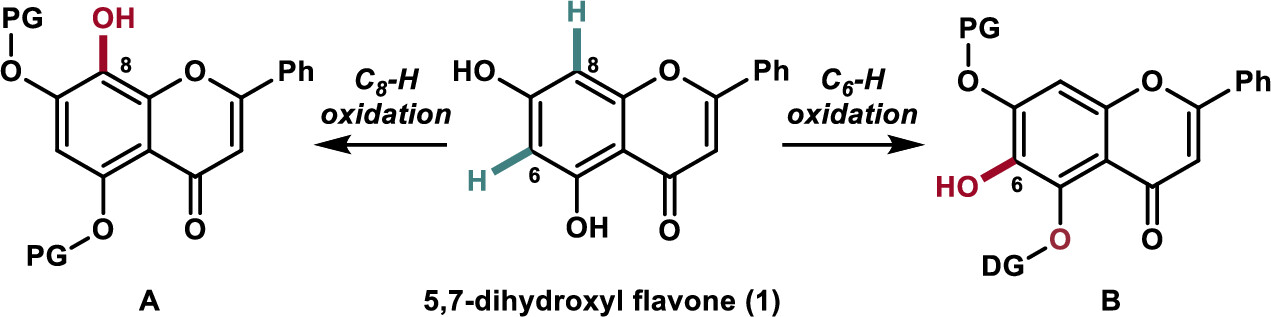
Regioselective synthesis of 5,6,7-trihydroxyl and 5,7,8-trihydroxyl flavones has been achieved via a transition-metal-catalyzed C–H oxidation as the key step using naturally enriched 5,7-dihydroxyl flavone. The developed chemistry was applied to the synthesis of the naturally occurring and biologically active flavonoids wogonin (2), oroxylin A (3), and their glycosylated derivatives (4 and 5) as potential carnitine palmitoyltransferase 1 activators.