88.Asymmetric total synthesis of (−)-lingzhiol via a Rh-catalysed [3+2] cycloaddition
Rong Long, Jun Huang, Wenbin Shao, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
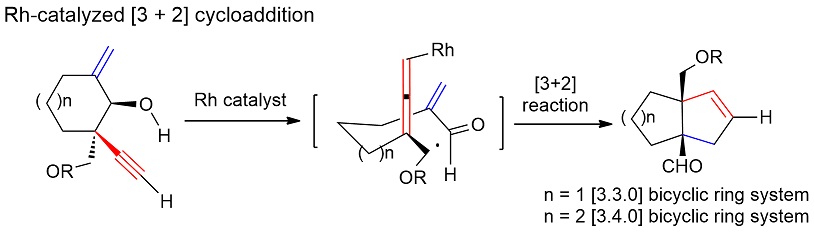
The development of efficient reactions for the one-pot construction of bicyclic ring systems bearing two quaternary carbon centres at their bridgehead positions represents a significant challenge to synthetic chemistry. The development of new methods capable of overcoming this challenge is highly desirable, because this motif can be found in a wide range of natural products with significant biological activities. Herein, we report an efficient [3+2] cycloaddition reaction between an enal and an alleno rhodium species, which was generated in situ from the corresponding enynol via a retro metal-propargylation reaction, to give [3.3.0] and [3.4.0] bicyclic systems bearing two quaternary atoms at their bridgehead positions. The developed chemistry has been successfully applied to the asymmetric total synthesis of natural product (−)-lingzhiol (4) for the first time in 17 steps.