News&Event
89. Asymmetric Total Synthesis of (−)-Cebulactam A1
Shouliang Yang, Yumeng Xi, Jia-Hua Chen* and Zhen Yang*
Org. Chem. Front., 2014, 1, 91-99
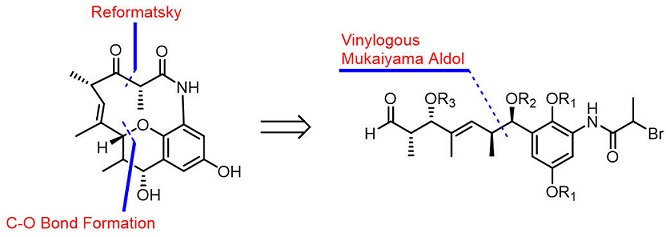
The total synthesis of (−)-cebulactam A1 (3) has been achieved for the first time in 18 steps. The key steps in this synthesis included an asymmetric chelation-controlled vinylogous Mukaiyama aldol reaction for the stereoselective synthesis of the stereogenic centers at the C8 and C9 positions, an intramolecular SmI2-mediated Reformatsky reaction for the formation of a macrocyclic lactam, and an SN2′ reaction for the stereoselective formation of the (E)-double bond linked tetrahydropyran moiety of cebulactam A1 (3).
88.Asymmetric total synthesis of (−)-lingzhiol via a Rh-catalysed [3+2] cycloaddition
Rong Long, Jun Huang, Wenbin Shao, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
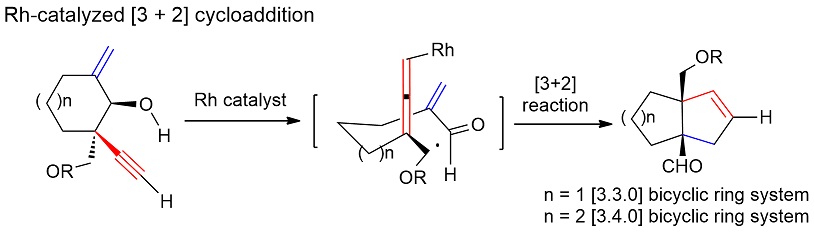
The development of efficient reactions for the one-pot construction of bicyclic ring systems bearing two quaternary carbon centres at their bridgehead positions represents a significant challenge to synthetic chemistry. The development of new methods capable of overcoming this challenge is highly desirable, because this motif can be found in a wide range of natural products with significant biological activities. Herein, we report an efficient [3+2] cycloaddition reaction between an enal and an alleno rhodium species, which was generated in situ from the corresponding enynol via a retro metal-propargylation reaction, to give [3.3.0] and [3.4.0] bicyclic systems bearing two quaternary atoms at their bridgehead positions. The developed chemistry has been successfully applied to the asymmetric total synthesis of natural product (−)-lingzhiol (4) for the first time in 17 steps.
87.Total synthesis of (+)-fusarisetin A
Jun Huang, Lichao Fang, Jianxian Gong, Chuangchuang Li, Zhen Yang*
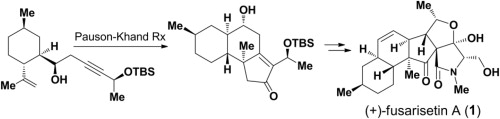
Herein we report the full details of our efforts toward the application of Pauson–Khand reaction for the stereoselective construction of the trans-decalin subunit with a C16 quaternary stereocenter of fusarisetin A, which led to the asymmetric total synthesis of (+)-fusarisetin A. The developed chemistry provides an alternative strategy to the intramolecular Diels–Alder reaction that has been employed for the synthesis of trans-decalin based natural products.
Congratulation to Hongjuan Shen, Jingjie Li and Junkai Fu for their works were published on OL and CEJ respectively!
Recently, Hongjuan Shen, Jingjie Li and Junkai Fu’s works were published on Org Lett and Chem. Eur. J respectively!
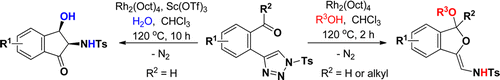
Miss Hongjuan Shen’s work represented two novel methods to synthesis of dihydroisobenzofuran and indanone derivatives from 2-triazole-benzaldehydes and 2-triazole-alkylaryl ketones via rhodium(II)-catalyzed tandem reactions.
For more details, please see the paper online: https://web.pkusz.edu.cn/yang/86/ or Org. Lett., Article ASAP
Miss Jingjie Li’s work was published as an letter titled “Palladium-Catalyzed Oxidative Rearrangement of Tertiary Allylic Alcohols to Enones with Oxygen in Aqueous Solvent”. In her work, a one-pot procedure for Pd(TFA)2-catalyzed 1,3-isomerization of tertiary allylic alcohols to secondary allylic alcohols followed by a Pd(TFA)2/neocuproine-catalyzed oxidative reaction to β-disubstituted-α,β-unsaturated kenones was developed.
For more details, please see the paper online: https://web.pkusz.edu.cn/yang/85/ or Org. Lett., 2014, 16, 5370
Mr. Junkai Fu’s work was focused on the 4-substituted-1-tosyl-1,2,3-triazole-based stereoselective synthesis of structurally diverse oxaspirocycles. The synthesis involves Rh-catalyzed loss of nitrogen from 4-substituted-1-tosyl-1,2,3-triazoles, Grignard reaction, and a ring-closing metathesis reaction as key steps.
For more details, please see the paper online: https://web.pkusz.edu.cn/yang/84/ or Chem. Eur. J. 2014, 20, 12881
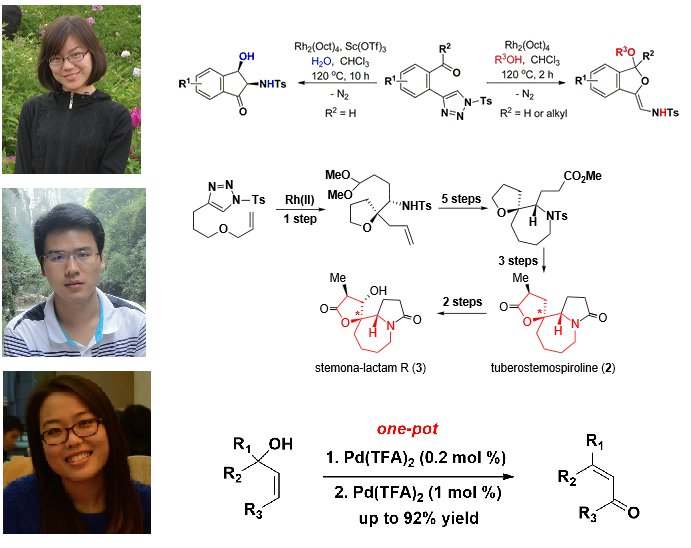
86.Tunable and Chemoselective Syntheses of Dihydroisobenzofurans and Indanones via Rhodium-Catalyzed Tandem Reactions of 2‑Triazole-benzaldehydes and 2‑Triazole-alkylaryl Ketones
Hongjuan Shen, Junkai Fu, Jianxian Gong,* and Zhen Yang*
Two novel rhodium(II)-catalyzed tandem reactions were developed for the synthesis of dihydroisobenzofuran and indanone derivatives from 2-triazole-benzaldehydes and 2-triazole-alkylaryl ketones. Dihydroisobenzofuran derivatives were obtained in good yields with high regioselectivities when alcohols were used as nuclophiles in these reactions, whereas the replacement of the alcohol with water resulted in the diastereoselective formation of highly functionalized indanone derivatives.