Publications
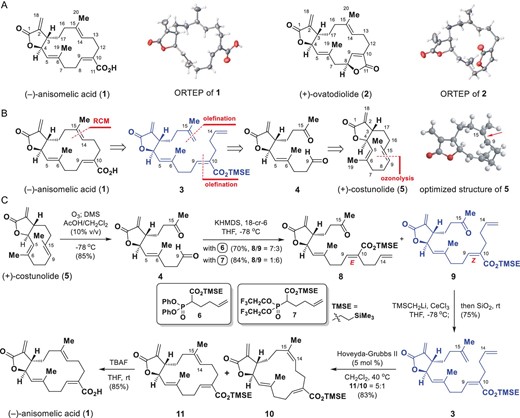
165.Identification and semisynthesis of (−)-anisomelic acid as oral agent against SARS-CoV-2 in mice
Hai-Xin Yu, Nan Zheng, Chi-Tai Yeh, Chien-Ming Lee, Qi Zhang, Wen-Lv Zheng, Qing Chang, Yuan-He Li, Yu-Jun Li, Gui-Zhen Wu∗, Jun-Min Quan∗, Lin-Qi Zhang∗, Yew-Min Tzeng∗ and Zhen Yang∗
National Science Review 2022, 9(11), nwac 176
(−)-Anisomelic acid, isolated from Anisomeles indica (L.) Kuntze (Labiatae) leaves, is a macrocyclic cembranolide with a trans-fused α-methylene-γ-lactone motif. Anisomelic acid effectively inhibits SARS-CoV-2 replication and viral-induced cytopathic effects with an EC50 of 1.1 and 4.3 μM, respectively. Challenge studies of SARS-CoV-2-infected K18-hACE2 mice showed that oral administration of anisomelic acid and subcutaneous dosing of remdesivir can both reduce the viral titers in the lung tissue at the same level. To facilitate drug discovery, we used a semisynthetic approach to shorten the project timelines. The enantioselective semisynthesis of anisomelic acid from the naturally enriched and commercially available starting material (+)-costunolide was achieved in five steps with a 27% overall yield. The developed chemistry provides opportunities for developing anisomelic-acid-based novel ligands for selectively targeting proteins involved in viral infections.
164.Electrocatalytic Isomerization of Allylic Alcohols: Straightforward Preparation of β-Aryl-Ketones
Anding Li, Nan Zheng, Kai Guo, Zhongchao Zhang and Zhen Yang*
Electrochemical synthesis has been rapidly developing over the past few years. Here, we report a practical and eco-friendly electrocatalytic isomerization of allylic alcohols to their corresponding carbonyl compounds. This reaction can be carried out in undivided cells without the addition of external chemical oxidants and metal catalysts. Moreover, this reaction features a broad substrate scope including challenging allylic alcohols bearing tri- and tetra-substituted olefins and affords straightforward access to diverse β-aryl-ketones. Mechanistic investigations suggest that the reactions proceed through a radical process. This study represents a unique example in which electrochemistry enables hydrogen atom transfer in organic allylic alcohol substrates using a simple organocatalyst.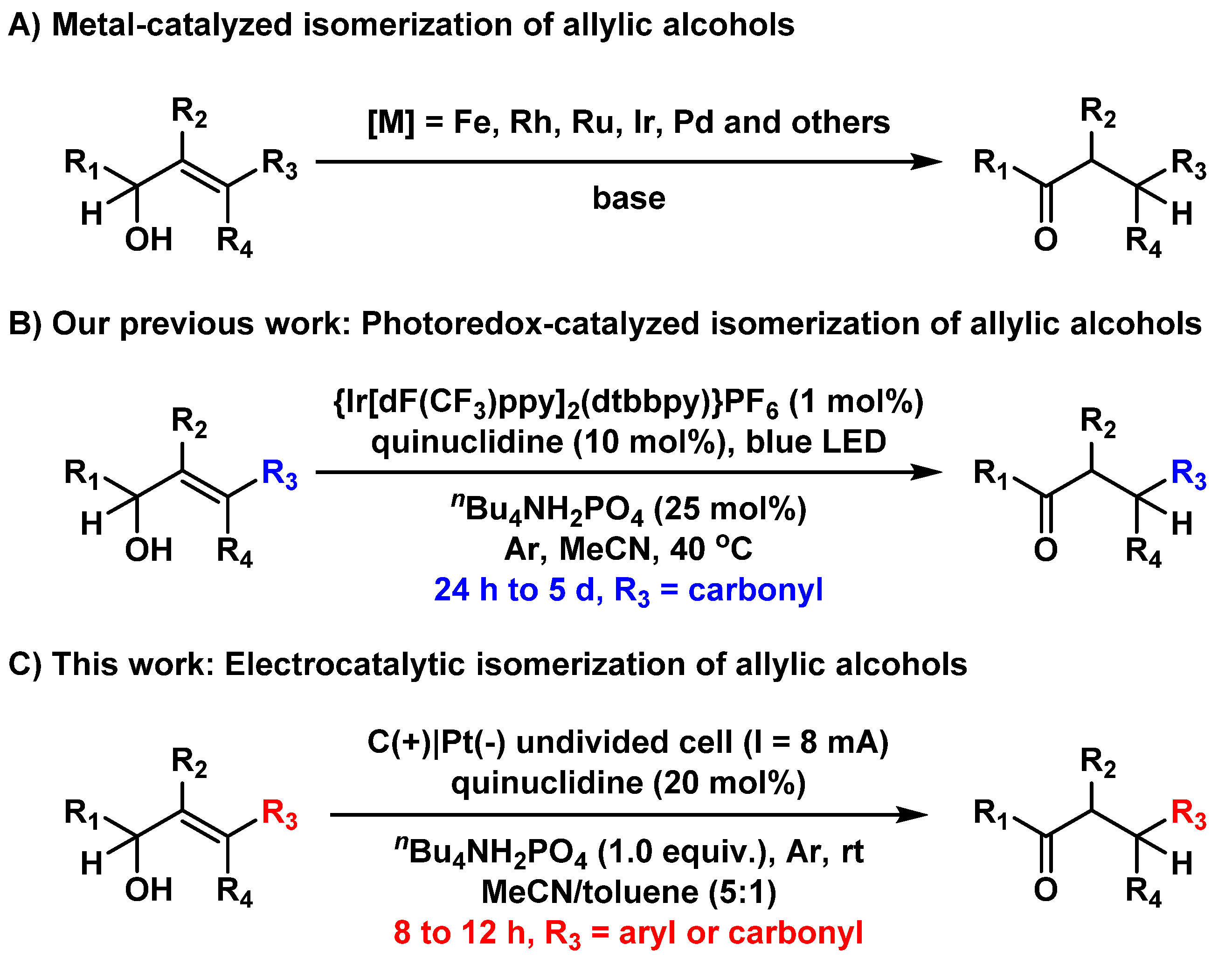
163.Semisynthesis of (−)-Bufospirostenin A Enabled by Photosantonin Rearrangement Reaction
Jun Huang,* Tingting Cao, Zhongchao Zhang, and Zhen Yang*
J. Am. Chem. Soc. 2022, 144,(6), 2479–2483
An enantioselective semisynthesis of (−)-bufospirostenin A is described. The key steps in the synthesis involve use of our proposed biomimetic and diastereoselective photosantonin rearrangement reaction for construction of the 5/7 bicyclic motif, and a Co-catalyzed reversible double-bond isomerization reaction for installing the double bond in the seven-membered ring.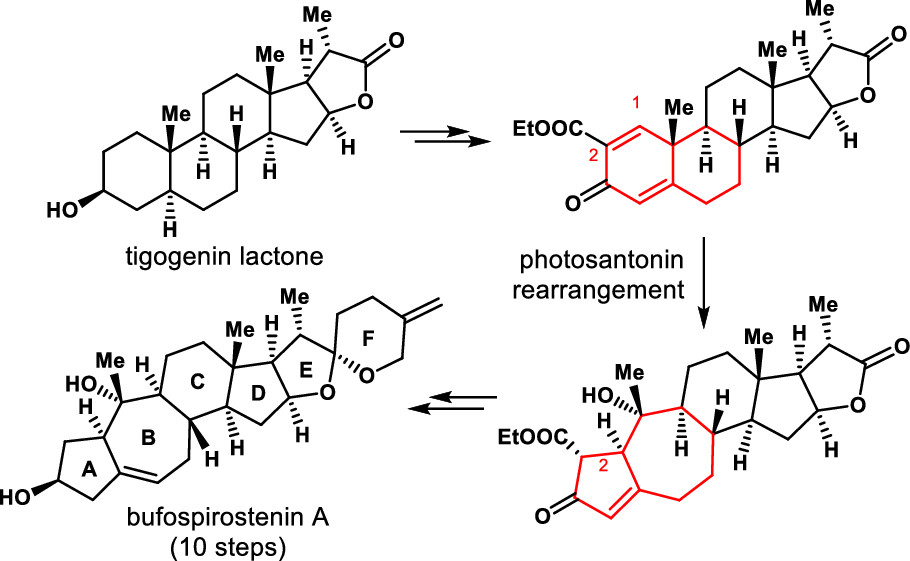
162.Palladium-Catalyzed Intramolecular Diarylation of 1,3-Diketone in Total Synthesis of (±)-Spiroaxillarone A
Tingting Cao, Lei Zhu,* Jun Huang,* and Zhen Yang*
Org. Lett. 2022, 24,(7), 1491–1495
A sterically congested all-carbon quaternary center was installed for the first time via a Pd-catalyzed cascade diarylation with aryl bromides and acyclic 1,3-diketones. This method was used as a key step in the total synthesis of (±)-spiroaxillarone A. Computational experimental results indicated that the selective diarylation is accelerated by the higher free-energy barriers of the endothermic transmetalation and reductive elimination in the first arylation step.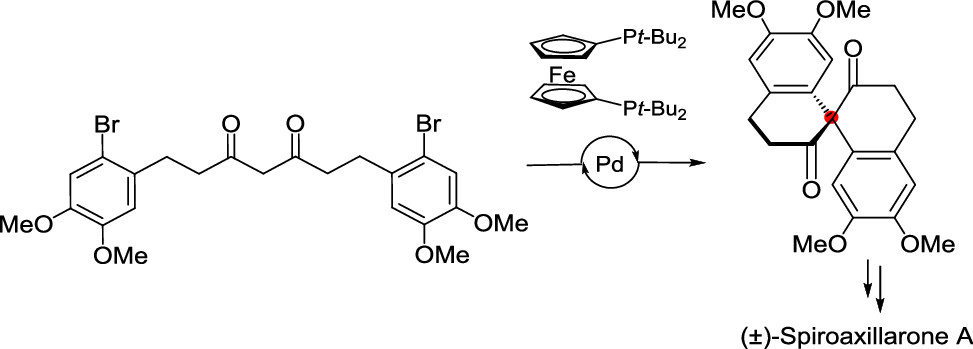
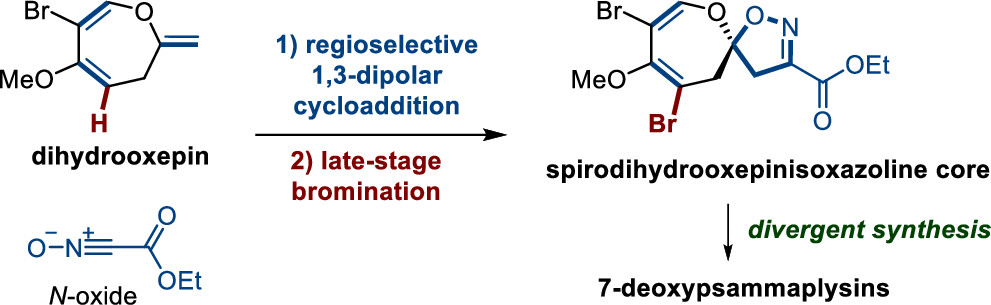
161.Concise Synthesis of 7-Deoxypsammaplysins K and O and 7-Deoxyceratinamide A by 1,3-Dipole Cycloaddition
Lijie Zhang, Rongya Wang, Chao Wang, Bingyan Liu, Jinfeng Yang, Zhongchao Zhang, Jun Huang,*and Zhen Yang*
Org. Lett. 2022, 24(21), 3786–3791
A spiro-oxepin isoxazoline skeleton was constructed via 1,3-dipole cycloaddition as a key step, which enabled the total syntheses of 7-deoxyceratinamide A and 7-deoxypsammaplysins K and O. The developed chemistry could be applied to total synthesis of structurally diverse spiro-oxepin isoxazoline-based marine natural products.