Direct synthesis of amide or polyamides from alcohols and Amines with liberation of H2
Given the widespread importance of amides and polyamides in biochemical and chemical systems, an efficient synthesis that avoids wasteful use of stoichiometric coupling reagents or corrosive acidic and basic media is highly desirable.
In 2007, Milstein et. al. reported a reaction in which primary amines are directly acylated by equimolar amounts of alcohols to produce amides and molecular hydrogen (the only products) in high yields and high turnover numbers. (Science. 2007, 317, 790)
In 2011, Guan et. al. reported a direct synthesis of polyamides via catalytic dehydrogenation of diols and diamines on the basis of Milstein’s work. Polyamides with number average molecular weight from ∼10 to 30 kDa could be obtained from a wide variety of diols and diamines bearing aliphatic or aromatic, linear or cyclic spacers.
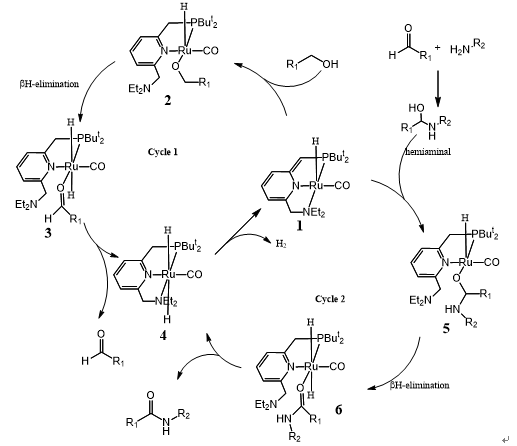
Milstein tentatively proposed the mechanism depicted in scheme 3 and Guan thought the process of polyamide formation followed a similar catalytic cycle. ( J. Am. Chem. Soc. 2011, 133, 1159).
The catalytic cycle begins with catalyst 1 coordination with alcohol and its aromatization, followed by -H elimination, aldehyde disassociation providing complex 4. Complex 4 gives out a molecular H2 and regenerates catalyst 1. The hemiaminal which formed form reaction aldehyde and amine can lead to the aromatic complex 5. -H elimination from 5 and followed ligand exchange can form the observed product amide and generated the known dihydride complex 4. Elimination of dihydrogen from complex 4 would regenerate catalyst 1, completing the catalytic cycle.
http://www.sciencemag.org/content/317/5839/790.short