Iridium-Catalyzed Borylation of Secondary Benzylic C−H Bonds Directed by a Hydrosilane
(3/6/2013, reported by Clark)
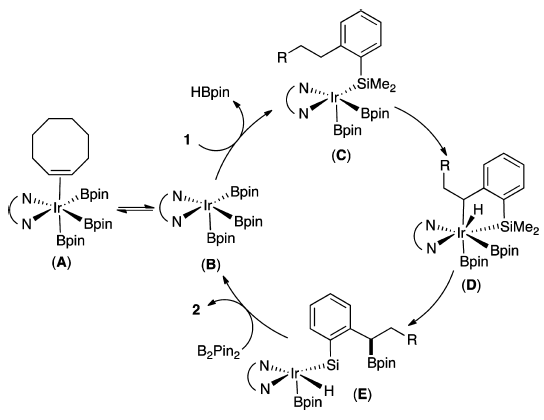
Most functionalizations of C−H bonds by main-group reagents occur at aryl or methyl groups. Hartwig and co-workers describe a highly regioselective borylation of secondary benzylic C−H bonds catalyzed by an iridium precursor and 3,4,7,8-tetramethyl-1,10-phenanthroline as the ligand. The reaction is directed to the benzylic position by a hydrosilyl substituent. This hydrosilyl directing group is readily deprotected or transformed to other functional groups after the borylation reaction, providing access to a diverse set of secondary benzylboronate esters by C−H borylation chemistry.
A proposed mechanism was given.
http://pubs.acs.org/doi/pdf/10.1021/ja403462b