Tao Cheng’s work has been published in Org. Lett.! Congratulations!
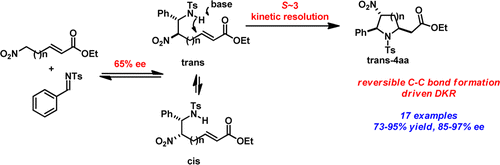
 A Highly Diastereoselective and Enantioselective Synthesis of Polysubstituted Pyrrolidines via an Organocatalytic Dynamic Kinetic Resolution Cascade
A Highly Diastereoselective and Enantioselective Synthesis of Polysubstituted Pyrrolidines via an Organocatalytic Dynamic Kinetic Resolution Cascade
Tao Cheng, Sixuan Meng, and Yong Huang*
Org. Lett., 2013, 15 (8), 1958–1961
Highly functionalized pyrrolidine and piperidine analogues, with up to three stereogenic centers, were synthesized in good yield (50–95%), excellent dr (single isomer), and high ee (>90%) using a Cinchona alkaloid-derived carbamate organocatalyst. High stereoselective synergy was achieved by combining a reversible aza-Henry reaction with a dynamic kinetic resolution (DKR)-driven aza-Michael cyclization. Whereas both reactions proceed with moderate enantioselectivities (50–60% for each step), high enantioselectivities are obtained for the overall products devoid of dr sacrifice.