Leming Wang and Jiean Chen’s work has been published in Angew. Chem. Int. Ed.!
Leming Wang, Jiean Chen and Yong Huang*, Angew. Chem. Int. Ed. 2015, 54, 15414.
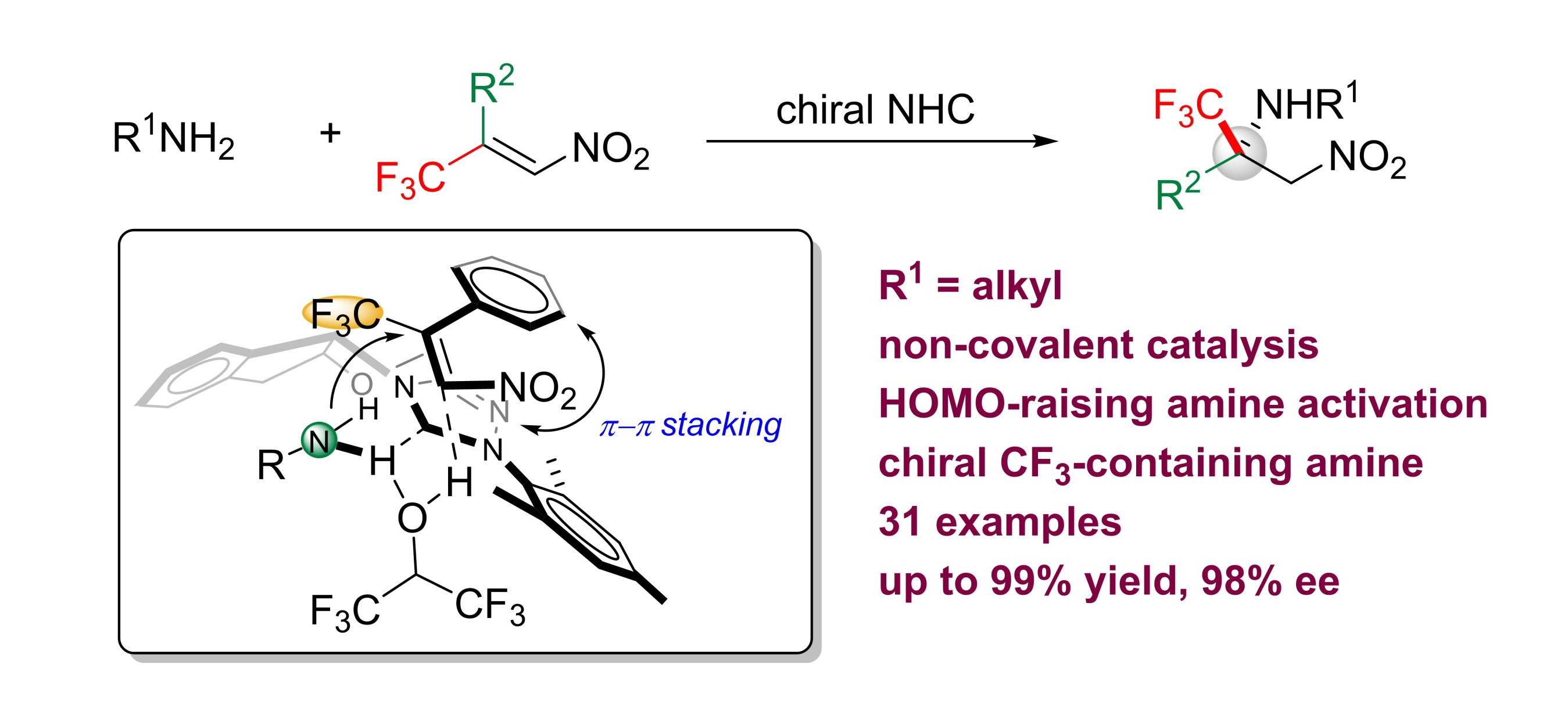
The aza-Michael addition reaction is a vital transformation for the synthesis of functionalized chiral amines. Despite intensive research, enantioselective aza-Michael reactions with alkyl amines as the nitrogen donor have not been successful. We report the use of chiral N-heterocyclic carbenes (NHCs) as noncovalent organocatalysts to promote a highly selective aza-Michael reaction between primary alkyl amines and β-trifluoromethyl β-aryl nitroolefins. In contrast to classical conjugate-addition reactions, a strategy of HOMO-raising activation was used. Chiral trifluoromethylated amines were synthesized in high yield (up to 99 %) with excellent enantioselectivity (up to 98 % ee).