Weiyu Yin’s work has been published in Adv.Synth.Catal.
Highly ortho-Selective Trifluoromethylthiolation Reactions using a Ligand Exchange Strategy
Weiyu Yin, ZhaofengWang and Yong Huang*, Adv. Synth. Catal. 2014, 356, 2998 – 3006
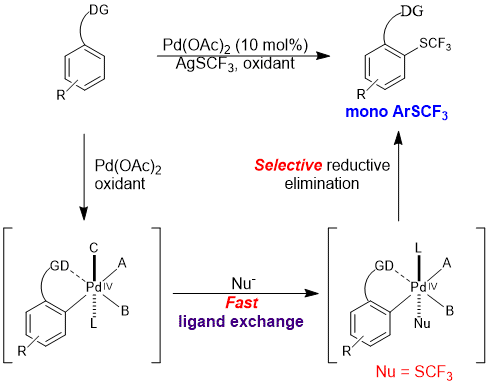
The trifluoromethylthio group (![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) SCF3) is a highly privileged modifier for drug molecules. Direct C
SCF3) is a highly privileged modifier for drug molecules. Direct C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H trifluoromethylthiolation reactions are highly valuable synthetic tools for the late-stage modification of drug candidates, which have so far been underexplored. We report a palladium-catalyzed ortho-selective mono-trifluoromethylthiolation reaction of arenes. The reaction proceeds through a key ligand exchange pathway using readily available silver trifluoromethylthiolate (AgSCF3). Acetic acid was found to be crucial to minimize oxidative dimerization of the starting materials and to facilitate the “SCF3−” transfer. We expect this strategy to have broad applications in C
H trifluoromethylthiolation reactions are highly valuable synthetic tools for the late-stage modification of drug candidates, which have so far been underexplored. We report a palladium-catalyzed ortho-selective mono-trifluoromethylthiolation reaction of arenes. The reaction proceeds through a key ligand exchange pathway using readily available silver trifluoromethylthiolate (AgSCF3). Acetic acid was found to be crucial to minimize oxidative dimerization of the starting materials and to facilitate the “SCF3−” transfer. We expect this strategy to have broad applications in C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) H functionalization reactions using a stand-alone oxidant and various anions.
H functionalization reactions using a stand-alone oxidant and various anions.