Wu group published article on Chem.Eur.J about a Preliminary Study of Diastereoselectivity in Pd(II)-catalyzed C(sp3)-H Alkoxylation of Cyclic system
The development of catalytic functionalization of C(sp3)–H bonds, especially unactivated methylene C(sp3)–H bonds, is still far lag behind that of C(sp2)-H bonds. Compared to the activation of C(sp2)-H bonds and primary C(sp3)–H bonds, methylene C(sp3)-H bonds activation is more challenging due to several factors such as intrinsic inertness, steric hindrance and competing β-hydride elimination. Besides, generation of stereoisomers also complicates the methylene C–H bonds activation process.
Aliphatic cyclic compounds bearing special chemical space are important motifs occurring in many natural products and drugs. As the mechanism details about the origin of diastereoselectivity remain unclear, a better understanding of the mechanism will be helpful for controlling stereoselectivity in C-H activation reaction of both cyclic and acyclic systems.
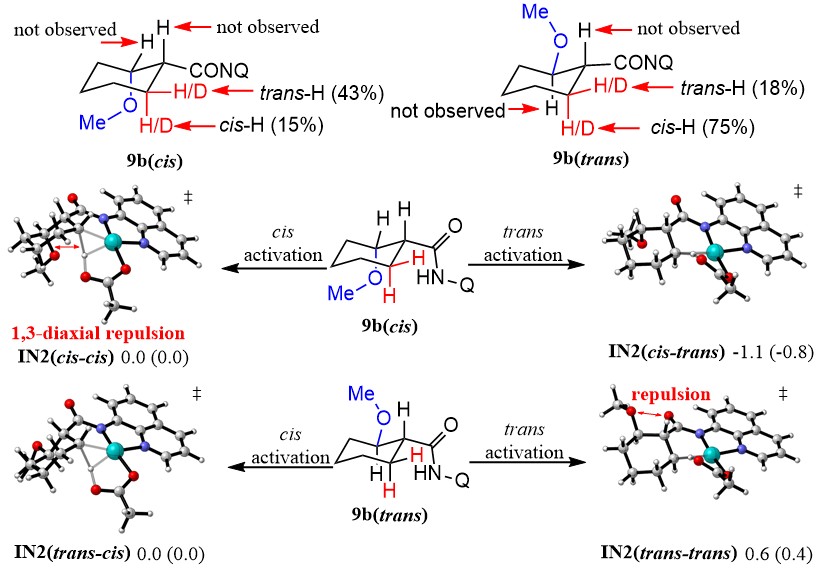
The primary mechanism of the Pd(II)-catalyzed 8-aminoquinoline directed C-H alkoxylation was investigated. It was believed that Pd(II)-catalyzedC(sp3)-O bond formation is through concerted reductive elimination from Pd(IV) intermediate in cyclic system. Deuteration experiments and related computational studies elucidated that intrinsic conformation determined the diastereoselectivity of Pd(II)-catalyzed C-H alkoxylation of cyclic carboxylic acids.
The Ph.D. student Tian-Yu Sun from Wu group is the second author of this paper. This article was published in the “Chemistry A European Journal” (Xinglin Yang.; Tianyu Sun.; Yu Rao., A Preliminary Study of Diastereoselectivity in Pd(II)-catalyzed C(sp3)-H Alkoxylation of Cyclic system. Chemistry A European Journal, DOI: 10.1002/chem.201503967).
Linking: http://pubs.acs.org/doi/abs/10.1021/acs.jpcb.5b09027