Wu group published article on J. Org. Chem. about a mechanistic study on Ir-Catalyzed regio- and stereoselective hydrosilylation of internal thioalkynes
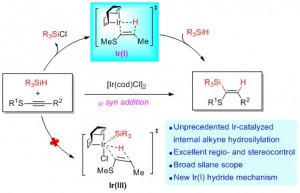
Iridium-complexes are known catalysts for a range of silylation reactions. However, the exploitation for selective hydrosilylation of unsymmetrical internal alkynes has been limitedly known. This article described a new example of this type. Specifically, [(cod)IrCl]2 catalyzes the efficient and mild hydrosilylation of thioalkynes by various silanes with excellent regio- and stereoselectivity.
DFT studies suggested a new mechanism for this reaction. Unlike the classic Chalk−Harrod mechanism using Ir(III) hydride as the key intermediate, it was found that a mechanism involving an Ir(I) hydride intermediate is most consistent with the observed mild conditions and excellent stereo- and regiocontrol. The Ir(I) hydride intermediate generating from reductive elimination of Si(OMe)3Cl provides enough space for alkyne coordination. The subsequent hydrometalation forms a sulfur-chelated intermediate, which determines both stereo- and regioselectivity. The following oxidative addition and final reductive elimination yields the final cis-addition product.
The Ph.D. student Li-Juan Song from Wu group is the co-first author of this paper. This article was published in the “The Journal of Organic Chemistry”as a feature article (Li-Juan Song§, Shengtao Ding§, Yong Wang, Xinhao Zhang, Yun-Dong Wu and Jianwei Sun. Ir-Catalyzed Regio- and Stereoselective Hydrosilylation of Internal Thioalkynes: A Combined Experimental and Computational Study. http://pubs.acs.org/doi/abs/10.1021/acs.joc.6b00854).