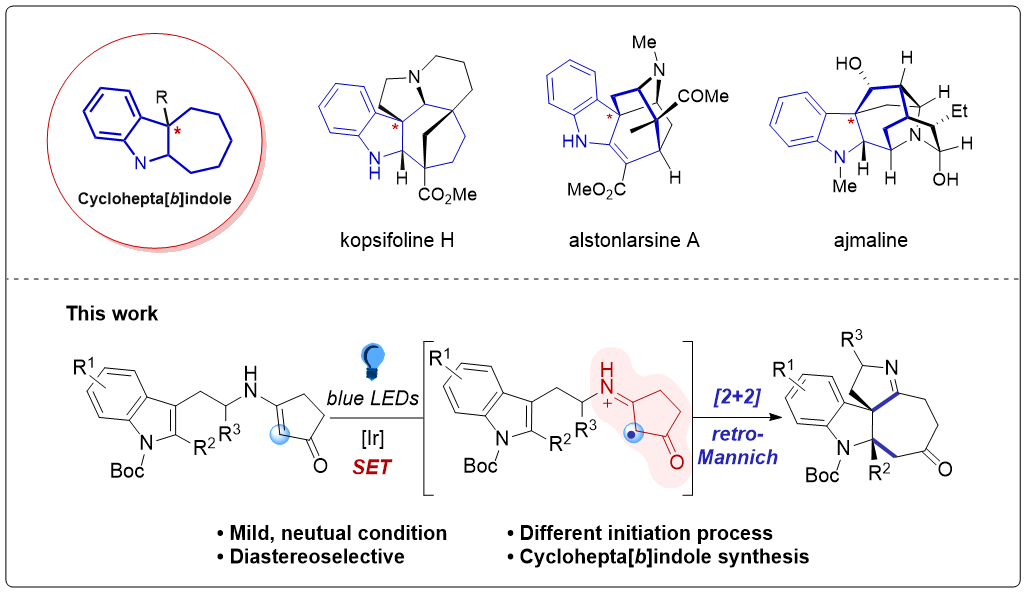
153.Stereoselective Synthesis of Cyclohepta[b]indoles via Visible‐Light‐Induced [2+2]‐Cycloaddition/retro‐Mannich‐type Reactions
Xin-Peng Mu, Yuan-He Li, Nan Zheng, Jian-Yu Long, Si-Jia Chen, Bing-Yan Liu, Chun-Bo Zhao, and Zhen Yang*
A novel method for the concise synthesis of cyclohepta[ b ]indoles in high yields was developed. The method involves a visible‐light‐induced, photocatalyzed [2+2]‐cycloaddition / retro‐Mannich‐type reaction of enaminones. Experimental and computational studies suggested that the reaction is a photoredox process initiated by single‐electron oxidation of an enaminone moiety, which undergoes subsequent cyclobutane formation and rapidly fragmentation in a radical‐cation state to form cyclohepta[ b ]indoles.