Publications
75.Asymmetric Total Synthesis of Caribenol A via an Intramolecular Diels−Alder Reaction
Jing-Chun Han, Lian-Zhu Liu, Yuan-Yuan Chang, Guo-Zong Yue, Jie Guo, Li-Yan Zhou, Chuang-Chuang Li*, and Zhen Yang*
-
• Highlighted in Synfacts, 2013, 9, 809.
• Highlighted in Org. Chem. Highlights, December, 2013.
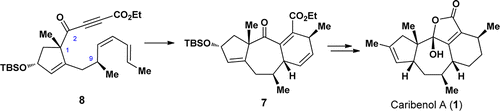
A total synthesis of the caribenol A (1), a novel natural product with an intriguing tetracyclic framework, has been achieved. The synthesis features an intramolecular Diels–Alder (IMDA) reaction for the facile construction of the tricyclic [5–7–6] skeleton of caribenol A (1) and a biomimetic oxidation reaction for the formation of the 2-hydroxyfuran-2(5H)-one motif of caribenol A (1) as key steps. This synthetic approach also reveals that the sp2 carbon at C(2) in substrate 8 is a critical factor for the formation of the tricyclic [5–7–6] skeleton in 7.
74.Highly Regioselective Syntheses of Substituted Triphenylenes from 1,2,4-Trisubstituted Arenes via a Co-Catalyzed Intermolecular Alkyne Cyclotrimerization
Lingmin Xu, Ruocheng Yu, Yuefan Wang, Jiahua Chen, and Zhen Yang
J. Org. Chem., 2013, 78 , 5744
Herein, we report the development of a new method for the syntheses of substituted triphenylenes from the corresponding 1,2,4-trisubstituted arenes, which were themselves generated in a highly regioselective manner according to an intermolecular alkyne cyclotrimerization reaction that was catalyzed by a novel Co–TMTU complex. This highly regioselective reaction for the formation of 1,2,4-trisubstituted arenes will be a valuable addition to the plethora of tools already available to synthetic chemists and encourage further mechanistic studies of this important alkyne trimerization process.

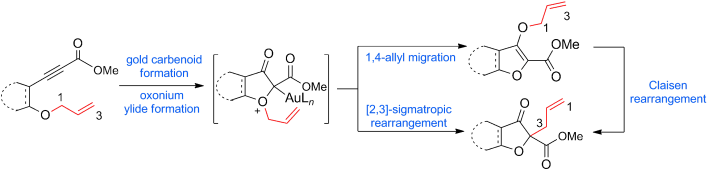
73.Gold-Catalyzed Rearrangement of Allylic Oxonium Ylides: Efficient Synthesis of Highly Functionalized Dihydrofuran-3-ones
Junkai Fu, Dr. Hai Shang, Zhaofeng Wang, Dr. Le Chang, Wenbing Shao, Prof. Dr. Zhen Yang, Prof. Dr. Yefeng Tang
Angew. Chem. Int. Ed. 2013, 52, 4198
“Diazo” not needed: The title reaction results in the rearrangement of oxonium ylides, which were prepared from readily available homopropargylic allylic ethers instead of diazo compounds, through two different mechanisms: a concerted 2,3-sigmatropic rearrangement, or a stepwise 1,4-allyl migration followed by a Claisen rearrangement (see scheme).
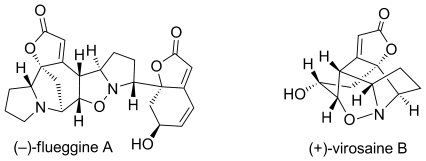
72.Stereoselective Total Syntheses of (−)-Flueggine A and (+)-Virosaine B
Dr. Hao Wei, Chuang Qiao, Gang Liu, Prof. Dr. Zhen Yang, Prof. Dr. Chuang-chuang Li
Angew. Chem. Int. Ed. 2013, 52, 2, 620
• Chosen as “Hot Paper” by the Editors.
• Top 3 Most Accessed Article in 11/2012.
• Highlighted in PKUSZ NEWS, 2012.
• Highlighted in Chemistry World, January, 2013.
• Highlighted in Synfacts, 2013, 9, 237 (“Synfact of the Month”).
Convergent approach: The total syntheses of (−)-flueggine A and (+)-virosaine B (see scheme) have been accomplished in a concise and convergent manner. Key steps in these approaches were relay ring-closing metathesis reactions for rapid construction of the key intermediates, and 1,3-dipolar cycloaddition reactions for the formation of the natural products.
71.Asymmetric Total Syntheses of Ansamacrolactams (+)-Q-1047H-A‑A and (+)-Q-1047H-R‑A
Shouliang Yang, Yumeng Xi, Rong Zhu, Lin Wang, Jiahua Chen, and Zhen Yang
The total syntheses of ansamacrolactams (+)-Q-1047H-A-A (16) and (+)-Q-1047H-R-A (17) have been achieved for the first time in 17 steps, leading to the reassignment of the relative stereochemistries and absolute configurations of their natural counterparts. The key steps in the synthetic work included an asymmetric chelation-controlled vinylogous Mukaiyama aldol reaction for the stereoselective synthesis of the syn-aldol adduct 7b and an intramolecular SmI2-mediated Reformatsky reaction for the formation of the macrocyclic lactam 14.






