Publications
160.Total Syntheses of Vicinal Dichloride Monoterpenes Enabled by AzaBellus−̌ Claisen Rearrangement
Jiangqun Cheng, Yuan-He Li, Jun Huang,* and Zhen Yang*
Org. Lett. 2021, 23(21), 8465–8470
Diastereoselective syntheses of syn– and anti-vicinal dihalides were achieved via an aza-Belluš–Claisen rearrangement, which involved the reaction of an α-chloro carboxylic acid chloride with halogen-substituted trans-allyl morpholines in the presence of Lewis acids. The developed method was used for the total synthesis of a group of monoterpene natural products bearing vicinal dichloride subunits.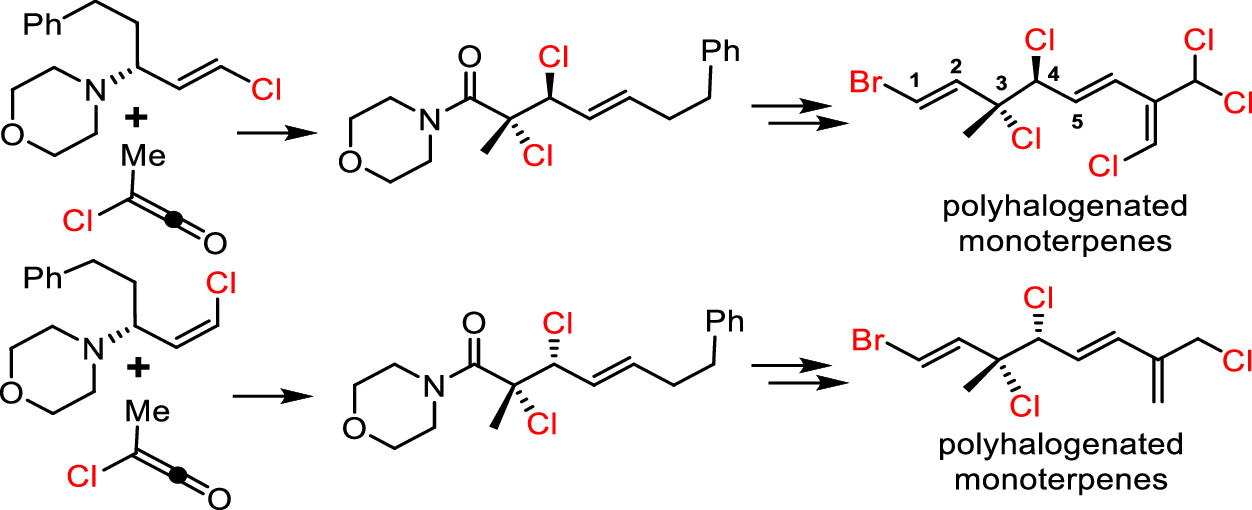
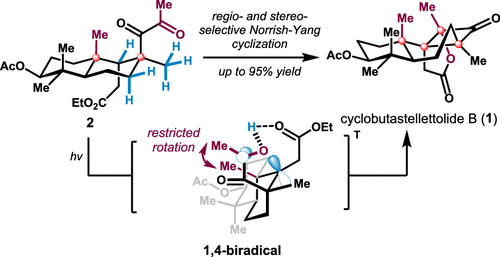
159.Total Synthesis of (+)-Cyclobutastellettolide B
Zhongchao Zhang, Sijia Chen, Fu Tang, Kai Guo, Xin-Ting Liang, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2021, 143,(43), 18287–18293
A convenient enantioselective total synthesis of (+)-cyclobutastellettolide B via a strategy that involves a diastereoselective Johnson–Claisen rearrangement, a regioselective cyclopropoxytrimethylsilane ring-opening reaction, and a Norrish–Yang cyclization is described. The results of computational and experimental studies indicate that the regio- and stereoselectivity of the Norrish–Yang reaction are controlled by the C–H bond dissociation energy and restricted rotation of the C13–C14 bond.
158.A Synthetic Route to The Core Structure of (−)-Retigeranic Acid A
Xiao Wang, Dian Li, Junlin Zhang, Jianxian Gong, Junkai Fu,* and Zhen Yang*
Org. Lett. 2021, 23,(13), 5092–5097
Retigeranic acid A is a uniquely structured pentacyclic sesterterpene bearing eight stereogenic centers. We report a concise route to the core structure of (−)-retigeranic acid A. The stereochemistry of its six chiral centers and three quaternary carbon centers was well-controlled. This route features two intramolecular Pauson–Khand reactions (IMPKRs): the first forged the D and E rings to deliver the triquinane subunit, and the second constructed the A and B rings and diastereoselectively installed the quaternary C6a center.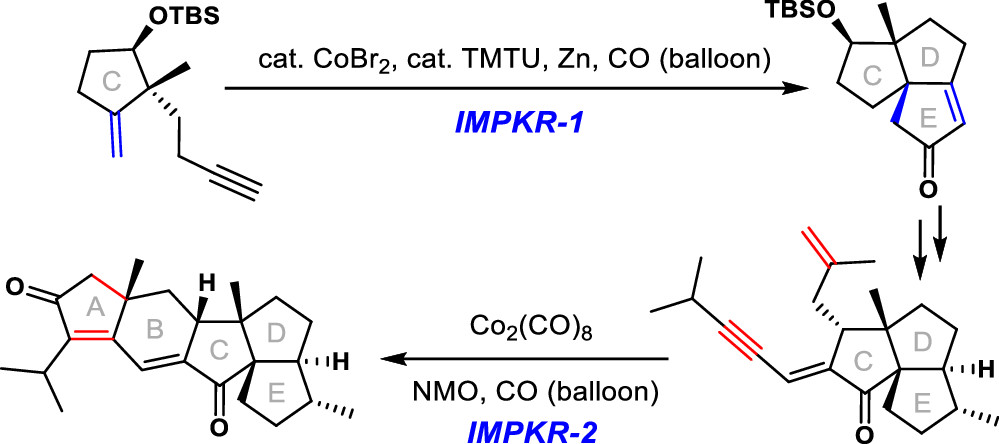
157.Stereoselective Synthesis of (±)-Cephanolide B
Anding Li, Ziru He, Bingyan Liu, Zhen Yang*, and Zichun Zhang*
Org. Lett. 2021, 23,(23), 9237–9240
A concise and stereoselective total synthesis of (±)-cephanolide B was achieved in 15 steps. The key steps in the synthesis were as follows: (i) an intermolecular Diels–Alder reaction followed by lactonization to form the oxabicyclo[2.2.2]octane DE ring; (ii) a tandem reaction, featuring an intramolecular Pauson–Khand reaction, a 6π-electrocyclization, and an oxidative aromatization by O2, to construct the ABC-tricyclic rings (6-5-6); and (iii) a phthaloyl peroxide-mediated arene oxygenation to install the C-13 phenol group.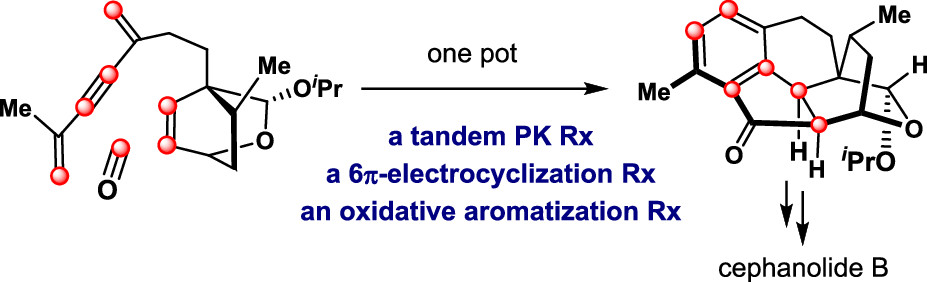
156.Synthetic Strategy for Construction of Highly Congested Tetracyclic Core (6–5–7–4) of Harziane Diterpenoids
Qian Tu, Zheyuan Wang, Zhongchao Zhang, Jun Huang*, and Zhen Yang*
Org. Lett. 2021, 23(11), 4088–4093
The structurally intriguing tetracyclic core of complex harziane diterpenoid was constructed in 14 steps from commercially available 3-ethoxycyclohex-2-en-1-one. The key steps were a Mn/Cu-mediated oxidative 1,3-dicarbonyl radical cascade cyclization reaction, which diastereoselectively formed the core of dimethylbicyclo[3.2.1]octane structure, and a Au-catalyzed diastereoselective formal [2 + 2] cycloaddition for construction of the harziane diterpenoid tetracyclic framework. The developed method paves the way for achieving total synthesis of this type of complex natural product.