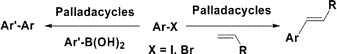Publications
10.Novel PdII-Mediated Cascade Carboxylative Annulation to Construct Benzo[b]furan-3-carboxylic Acids
Y. Liao*, J. Smith, R. Fathi*, Z. Yang*
Benzo[b]furan-3-carboxylic acid (2) was generated from 1 by forming three new bonds in one step via a PdII-mediated cascade carboxylative annulation. The proposed mechanism was supported by the observation of an unusual acetylation of 1 as a side reaction together with an 18O-labeling study.
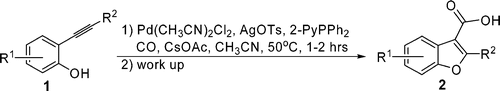
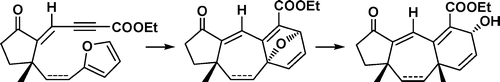
9.Exploring an Expedient IMDA Reaction Approach to Construct the Guanacastepene Core
C. C. Li. S. Liang, X. H. Zhang, Z. X. Xie, J. H. Chen*, Y. D.Wu*, Z. Yang*
Construction of the [5-7-6] tricyclic core of guanacastepenes was attempted by using the intramolecular Diels−Alder (IMDA) reaction and Me3Al-mediated ring opening of the oxabridge as key synthetic steps. The illustrated chemistry demonstrated a synthetic feasibility to build up the framework of guanacastepenes by the IMDA reaction.
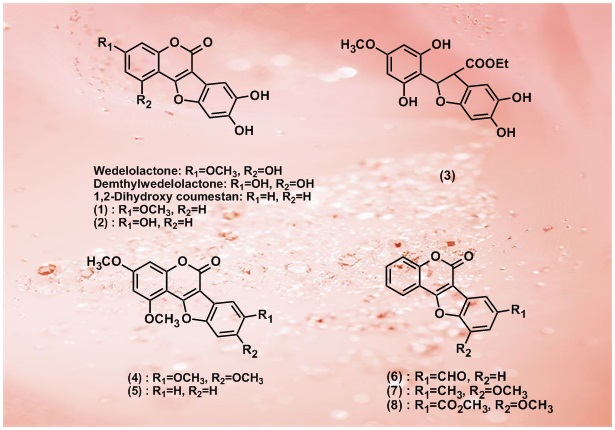
8.Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK Complex
M. Kobori, Z. Yang, V. Heissmeyer, H. Zhu, Y. K. Jung, M. A. M. Gakidis, A. Rao, T. Sekine, F. Ikegami, C. Yuan, J. Yuan
Cell Death and Differ. 2004, 11, 123.
Caspase-11 is a key regulator of proinflammatory cytokine IL-1 maturation and pathological apoptosis. Caspase-11 is not expressed in most tissues under normal condition, but highly inducible upon pathological stimulation such as in the presence of lipopolysaccharide (LPS). Here, we describe the identification and characterization of wedelolactone, a natural compound that inhibits LPS-induced caspase-11 expression in cultured cells by inhibiting NF-
maturation and pathological apoptosis. Caspase-11 is not expressed in most tissues under normal condition, but highly inducible upon pathological stimulation such as in the presence of lipopolysaccharide (LPS). Here, we describe the identification and characterization of wedelolactone, a natural compound that inhibits LPS-induced caspase-11 expression in cultured cells by inhibiting NF- B-mediated transcription. We demonstrate that wedelolactone is an inhibitor of IKK, a kinase critical for activation of NF-
B-mediated transcription. We demonstrate that wedelolactone is an inhibitor of IKK, a kinase critical for activation of NF- B by mediating phosphorylation and degradation of I
B by mediating phosphorylation and degradation of I B
B .
.
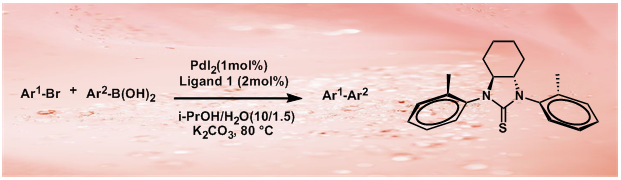
7.A Novel Thiourea Ligand Applied in the Pd-Catalyzed Heck, Suzuki, Suzuki Carbonylative Reactions
M. J. Dai, B. Liang, C. H. Wang, Z. J. You, J. Xiang, G. B. Dong, J. H. Chen*, Z Yang*
Adv, Synth. Catal. 2004, 346, 1669
A novel C2 symmetrical and sterically bulky thiourea ligand 1 has been successfully applied to Heck, Suzuki and Suzuki-type carbonylative coupling reactions under aerobic conditions. Since the metal-sulfur bond in the thiourea complexes is stronger than the metal-phosphorus bond of typical phosphine complexes, thiourea ligands generally do not easily dissociate from the metal center under catalytic conditions, which establishes the thiourea 1-based palladium complexes as effective catalysts for the palladium-catalyzed cross-coupling reactions.
6.Synthesis of Novel Palladacycles and Their Application in Heck and Suzuki Reactions under Aerobic Conditions
Z. C. Xiong, N. D. Wang, M. J. Dai, A. Li, J. H. Chen*, Z. Yang*
Design and synthesis of a novel family of furancarbothioamide-based palladacycles are reported herein. These palladacycles are thermally stable, not sensitive to air or moisture, and are applied effectively in the Heck reaction of aryl halides with terminal olefins and in the Suzuki reaction of aryl halides with arylboronic acids. These reactions were performed under aerobic conditions, leading to turnover numbers (TONs) up to 1 × 105.