Publications
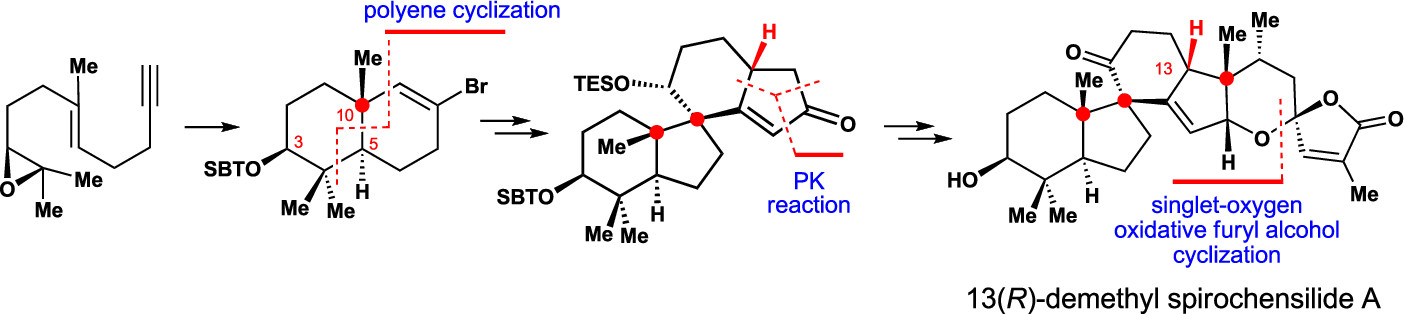
150.Asymmetric Total Synthesis of (−)-Spirochensilide A, Part 1: Diastereoselective Synthesis of the ABCD Ring and Stereoselective Total Synthesis of 13(R)-Demethyl Spirochensilide A
Xin-Ting Liang, Bao-Chuan Sun, Chang Liu, Yuan-He Li, Nan Zhang, Qian-Qian Xu, Zhong-Chao Zhang, Yi-Xin Han, Jia-Hua Chen,* and Zhen Yang*
J. Org. Chem. 2021, 86(3), 2135–2157
A concise and diastereoselective construction of the ABCD ring system of spirochensilide A is described. The key steps of this synthesis are a semipinacol rearrangement reaction to stereoselectively construct the AB ring system bearing two vicinal quaternary chiral centers and a Co-mediated Pauson–Khand reaction to form the spiro-based bicyclic CD ring system. This chemistry leads to the stereoselective synthesis of 13(R)-demethyl spirochensilide A, paving the way for the first asymmetric total synthesis of (−)-spirochensilide A.
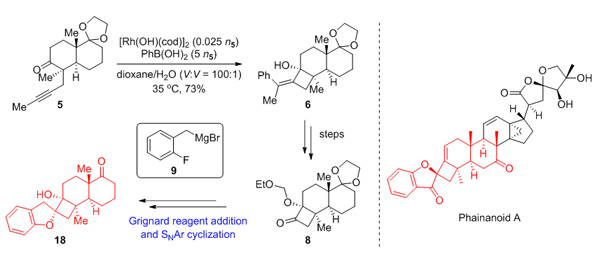
149.Synthetic Study Toward the 4,5-Spirocycle Skeleton of Phainanoids
Jiang, Chongguo†, Chen, Sijia†, Gong, Jianxian*, Yang, Zhen*
Acta Chim. Sinica 2020, 78(9), 928—932
Attempts to synthesize the 4,5-spirocycle skeleton of Phainanoids by rhodium-catalyzed arylative cyclization of alkynone 5 and the addition of Grignard reagent 9 to α-alkoxyl cyclobutone 8, followed by intramolecular SNAr reaction are reported. Phainanoids, highly modified triterpenoids, were isolated from Phyllanthus hainanensis by Yue and co-workers. They have been found to show intriguing immunosuppressive activities. The most potent of them, Phainanoid F, inhibit the proliferation of T cells with an IC50 value of (2.04±0.01) nmol/L and B cells with an IC50 value <(1.60±0.01) nmol/L. The noteworthy activities and the lack of Phainanoids in nature resources make the synthesis of them for further biological evaluation a challenge for chemists. Our synthesis started from known compound 1, after Birch reduction and alkylation to give alkynone 5. The rhodium-catalyzed arylative cyclization of alkynone 5 to deliver tetrasubstituted cyclobutene 6 was performed by the following procedure. Under an atmosphere of Ar, to an oven-dried Schlenk tube with[Rh(OH)(cod)]2 (35.5 mg, 0.078 mmol, 0.012n5), phenylboronic acid (2.0 g, 16.3 mmol, 2.5n5), were added a solution of ketone 5 (1.9 g, 6.5 mmol, 1.0n5) in 1,4-dioxane (32.0 mL) and H2O (0.3 mL) at room temperature. The mixture was stirred at 35℃ for 12 h. Another [Rh(OH)(cod)]2 (35.5 mg, 0.078 mmol, 0.012n5) and phenylboronic acid (2.0 g, 16.3 mmol, 2.5n5) was added to the mixture. The mixture was stirred at 35℃ for 12 h. Subsequently, hydroxyl group was protected with ethoxymethyl (EOM) group to furnish 7, followed by ozonolysis to generate ketone 8. Ketone 8 was reacted with fresh prepared Grignard reagent 9 in Felkin-Anh modelinstead ofthe Cram’s chelation-control model to deliver alcohol 10. The explanation of the diastereoselectivity of this reaction could be illustrated from two aspects:(1) the rigid structure of α-alkoxyl cyclobutone 8 increased the energy barrier for the transition state of chelation between magnesium ions and alkoxyl substituent; (2) the magnesium ions were not chelated with the alkoxyl substituent as well as the carbonyl oxygen was due to the intramolecular chelation with fluorine atom. Alcohol 10 underwent intramolecular SNAr reaction and deprotection to deliver 4,5-spirocycle compound 18.
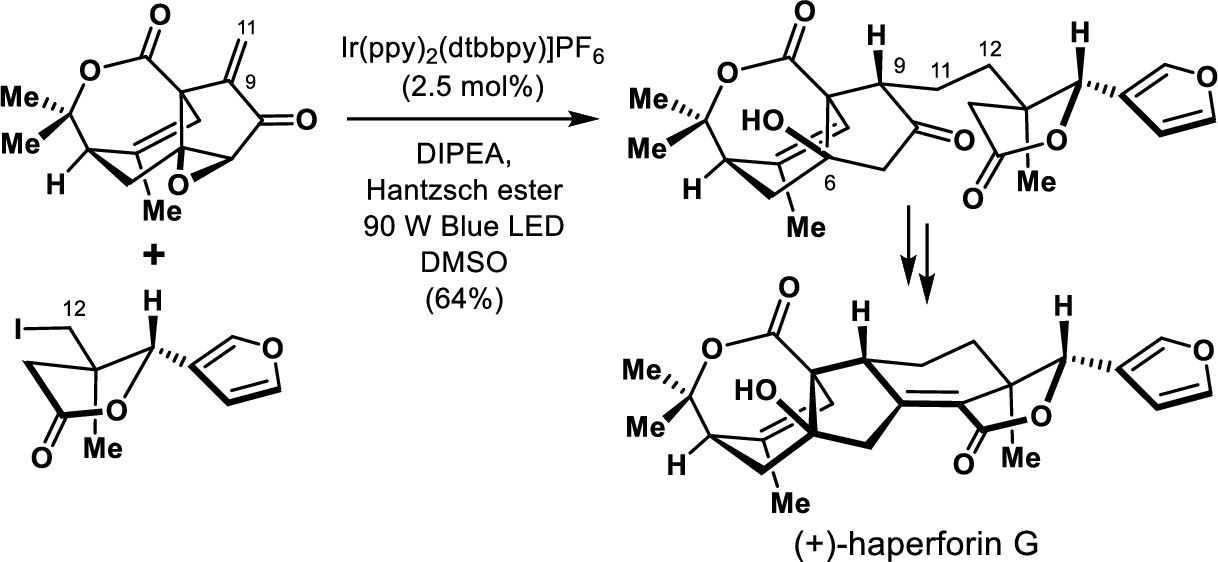
148.Total Synthesis of (+)-Haperforin G
Wei Zhang, Zhenyu Zhang, Jun-Chen Tang, Jin-Teng Che, Hao-Yu Zhang, Jia-Hua Chen,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(46), 19487–19492
A concise chemical synthesis of (+)-haperforin G in 20 steps from commercially available starting materials is achieved with the integration of the Co-catalyzed intramolecular Pauson–Khand reaction for the stereoselective construction of cyclopentanone bearing an all-carbon quaternary stereogenic center at the bridge-head position and the light-initiated photocatalysis for convergent and asymmetric cross-coupling of the unstabilized C(sp3)-radical with an enone. The developed chemistry paves the way to synthesizing structurally diverse analogs of haperforin G (6).
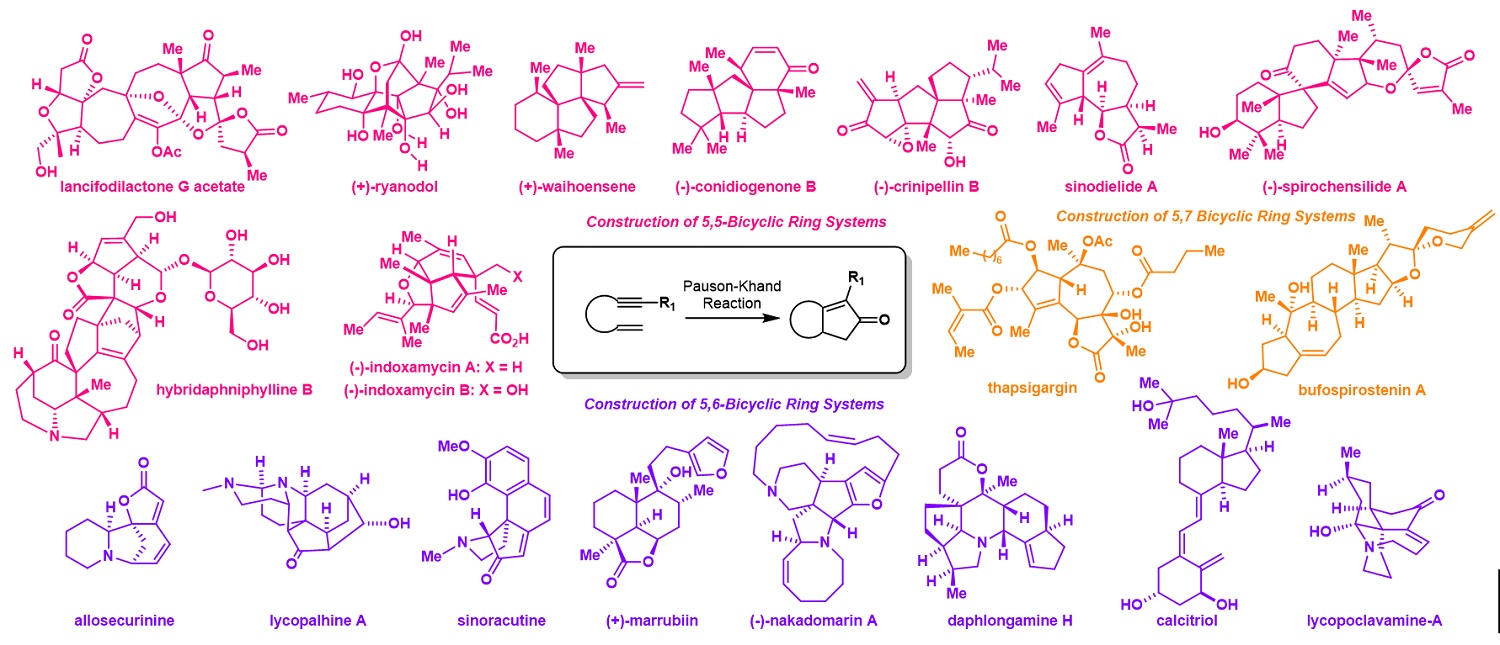
147.Evolution of Pauson-Khand Reaction: Strategic Applications in Total Syntheses of Architecturally Complex Natural Products (2016–2020)
Sijia Chen†, Chongguo Jiang†, Nan Zheng, Zhen Yang* and Lili Shi*
Catalysts, 2020, 10 (10), 1199
Metal-mediated cyclizations are important transformations in a natural product total synthesis. The Pauson-Khand reaction, particularly powerful for establishing cyclopentenone-containing structures, is distinguished as one of the most attractive annulation processes routinely employed in synthesis campaigns. This review covers Co, Rh, and Pd catalyzed Pauson-Khand reaction and summarizes its strategic applications in total syntheses of structurally complex natural products in the last five years. Additionally, the hetero-Pauson-Khand reaction in the synthesis of heterocycles will also be discussed. Focusing on the panorama of organic synthesis, this review highlights the strategically developed Pauson-Khand reaction in fulfilling total synthetic tasks and its synthetic attractiveness is aimed to be illustrated.
146.Synthesis of 4-Desmethyl-Rippertenol and 7-Epi-Rippertenol via Photoinduced Cyclization of Dienones
Zi-Chun Zhang†, Dan-Dan Zhao†, Zhong-Chao Zhang, Xin-Yu Tan, Jian-Xian Gong*, Jun-Kai Fu* & Zhen Yang*
Chinese Chemical Society, 2020, 2(5), 2074–2083
The synthesis of cycloheptanoid-based fused polycyclic frameworks is a challenge for organic chemists due to unfavorable entropic factors and ring strains. Herein, a concise synthesis of 4-desmethyl-rippertenol and 7-epi-rippertenol bearing a unique, [6,6,5,7]-fused tetracyclic framework is reported. The route features a novel photoinduced intramolecular cyclization of α-cyclopropyl dienone followed by an unexpected thermal 1,5-hydrogen migration, which provides efficient access to the fused seven-membered ring system in a stereochemically well-defined manner. Further density functional theory (DFT) calculations disclose that the stereoselectivity of this photoinduced process is mainly attributed to transition state conformation and steric effects.