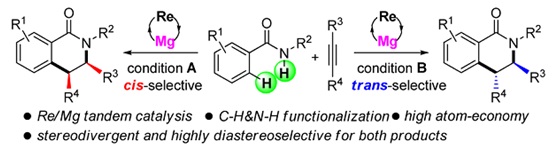
Re/Mg Bimetallic Tandem Catalysis for [4+2] Annulation of Benzamides and Alkynes via C‑H/N‑H Functionalization
(Reported by Dongqi Wang)
In the past few decades, C-H activation has attracted immense interest since it might significantly streamline the organic synthesis. The construction of isoquinolinone derivatives by using the C-H activation strategy is illustrative, but the redox-neutral annulation of benzamides with alkynes, one of the most straightforward routes to 3,4-dihydroisoquinolinones, remains an unmet challenge。
Recently, Wang et al. reported a rhenium-magnesium cocatalyzed [4+2] annulation of benzamides and alkynes via C-H/N-H functionalization. (J.Am.Chem.Soc., 2013, DOI:10.1021/ja400020e)
Plausible mechanism:
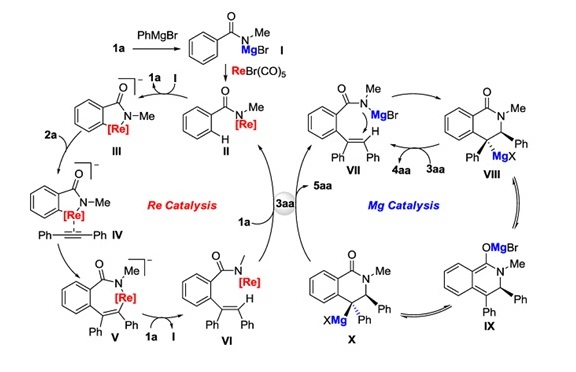
With the aid of PhMgBr, amido-rhenium II is initially formed via amido-magnesium I, which then undergoes adeprotonative cyclorhenation affording rhenacycle III. The ensuing coordination and insertion of an alkyne gives rise to seven-membered rhenacycle V, which further leads to inter-mediate VI upon protonation. Transmetalation between intermediate VI and substrate 1a results in the regeneration of amido-rhenium II and formation of 3aa, which after deprotonation, suffers an intramolecular nucleophilic addition/cyclization generating species VIII or further leading to X via intermediate IX. Protonation of these species by 3aa would afford the final products and regenerate amido-magnesium VII, thus closing the entire Re/Mg bimetallic tandem catalytic cycles.
