RhIII/CuII-Cocatalyzed Synthesis of 1H Indazoles through C−H Amidation and N−N Bond Formation
Due to their diverse pharmacological activities, 1H-indazoles are widely used in clinical medicines. Traditional methods to synthesize 1H-indazoles are suffer from several disadvantages, such as harsh reaction conditions, expensive starting materials, or carcinogenic reaction partners must be used.
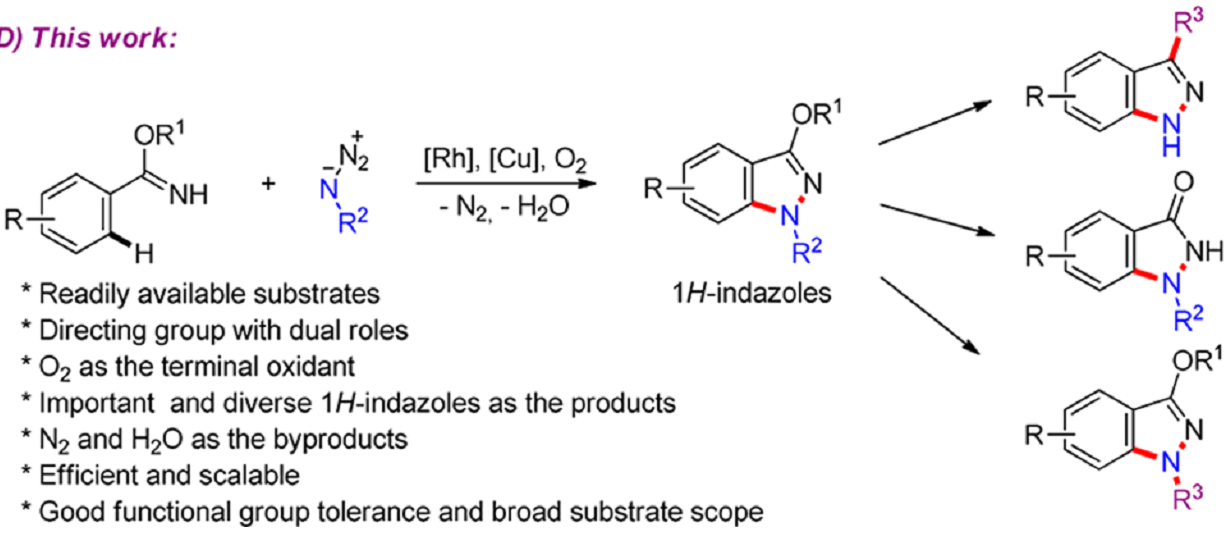
Recently, Frank Glorius and his coworkers developed an attractive process to synthesize 1H-indazoles byRhIII/CuII-Cocatalyzed Synthesis of 1H‑Indazoles through C−H Amidation and N−N Bond Formation.
The reaction use easily available arylimidates and organo azides as starting material, and it’s the first time N-H-Imidates were applied as directing groups in RhIII-catalyzed C−H activation, delivering substrates that undergo intramolecular N−N bond formation. The process is also scalable and green, with O2 being used as the terminal oxidant and only N2 and H2O formed as the byproducts. The products could also be further transformed to diverse important derivatives.
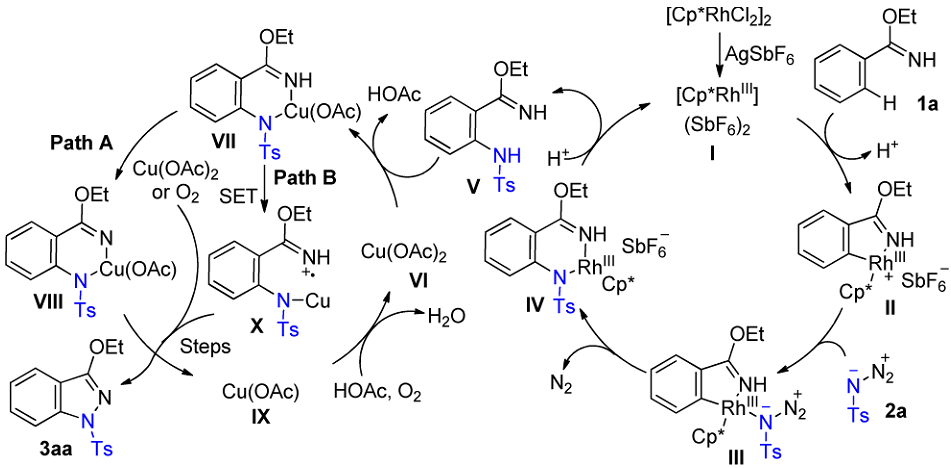
Plausible mechanism:
[PDF]