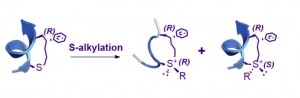
 Modification of the cross-linker of constrained peptides has recently received considerable attention. Here, we present a versatile approach to modify the cross-linking tether of chiral center-induced helical (CIH) peptides via S-alkylation reac-tion. The alkylation process displayed high conversion efficiency, selectivity and substrate tolerance. Notably, although on-tether S-alkylation could lead to a pair of peptide epimers, the major alkylated product retained the helical structure of its helical precursor peptide. This S-alkylation was readily reversible under reductive conditions, which provides a sim-ple method for traceless modification. In addition to expanding the chemical space of CIH peptides, this strategy is the first on-tether modification platform with known retention of the peptides’ original helicity.
Modification of the cross-linker of constrained peptides has recently received considerable attention. Here, we present a versatile approach to modify the cross-linking tether of chiral center-induced helical (CIH) peptides via S-alkylation reac-tion. The alkylation process displayed high conversion efficiency, selectivity and substrate tolerance. Notably, although on-tether S-alkylation could lead to a pair of peptide epimers, the major alkylated product retained the helical structure of its helical precursor peptide. This S-alkylation was readily reversible under reductive conditions, which provides a sim-ple method for traceless modification. In addition to expanding the chemical space of CIH peptides, this strategy is the first on-tether modification platform with known retention of the peptides’ original helicity.
Link: http://pubs.acs.org/doi/10.1021/acs.bioconjchem.7b00321