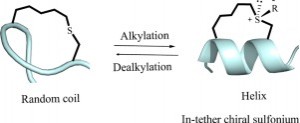
 A chirality induced helicity method has been developed to modulate the peptide’s biophysical and biochemical properties. We report herein a novel approach for reversibly switching the conformation of short constraint α-helical peptides through alkylation of the in-tether thioether and dealkylation of the chiral sulfonium. This traceless redox sensitive tagging strategy broadened our scope of CIH (chirality induced helicity) strategy and provided a valuable approach to functionalize the peptide tether
A chirality induced helicity method has been developed to modulate the peptide’s biophysical and biochemical properties. We report herein a novel approach for reversibly switching the conformation of short constraint α-helical peptides through alkylation of the in-tether thioether and dealkylation of the chiral sulfonium. This traceless redox sensitive tagging strategy broadened our scope of CIH (chirality induced helicity) strategy and provided a valuable approach to functionalize the peptide tether
Link: http://www.sciencedirect.com/science/article/pii/S1001841717302395