Research
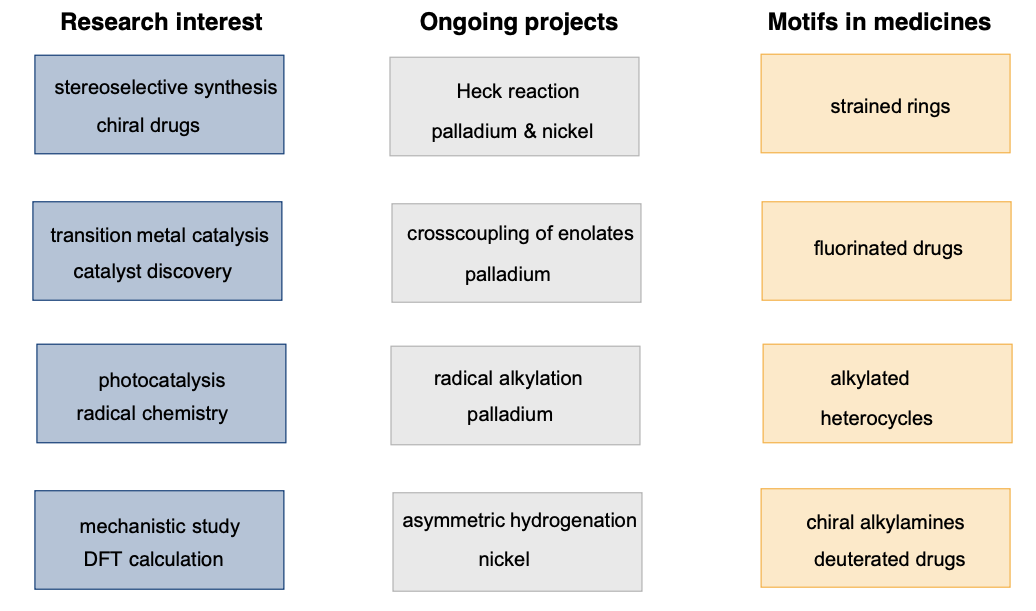
My research focuses on transition metal catalysis, mechanistic studies and application to stereoselective synthesis of chiral drugs. An emphasis is placed on asymmetric catalytic processes. Chiral drugs contribute to one third of modern medicines today. Nowadays almost all of newly approved small-molecule drugs are made in optically pure forms, so as to avoid toxicity caused by unwanted isomers.
Other research interest includes: 1) Application of photocatalysis and reactive radical species in metal catalysis. 2) Replacement of expensive noble metals with Earth-abundant late 3d metals. 3) Stereoselective formation of strained rings, deuterated and fluorinated compounds, which are becoming increasingly important in medicines.
Examples of ongoing projects include 1) Heck reaction, 2) arylation of carbonyl compounds, 3) palladium radical catalysis, 4) nickel-catalyzed transfer hydrogenation and 5) copper-catalyzed addition of air-stable organoboron reagents. In particular, asymmetric hydrogenation has become an important tool for large-scale synthesis of chiral key intermediates in industry, but hydrogenation catalysts rely heavily on very expensive, rare metals, such as rhodium, iridium and ruthenium. It poses a severe problem of sustainability in pharmaceutical manufacturing.
I also seek intellectual contribution to transition metal catalysis at a fundamental level: 1) New ways of bond activation, for example, using classical hydrogen bonding for soft ionization of arylpalladium halides in asymmetric Heck reaction. 2) New ways of bond formation in metal catalysis, for example, discovery of a new elementary step of 1,4-insertion in copper-catalyzed asymmetric addition. 3) New means of chirality transfer from catalysts to products, for example, employing weak CH/O hydrogen bonding in palladium-catalyzed asymmetric coupling of enolates.

