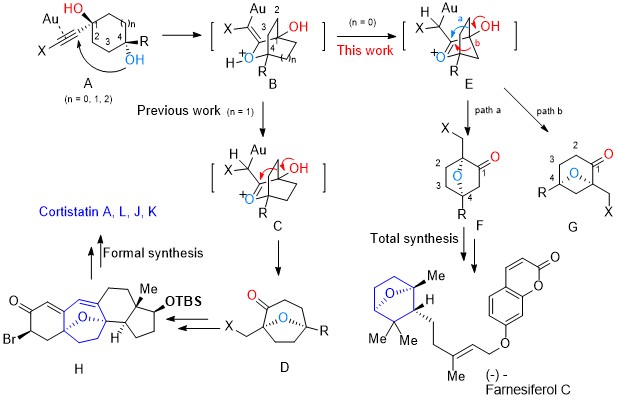
101.Regio- and Stereoselective Syntheses of 7-Oxabicyclo[2.2.1]heptanes via a Gold(I)-Catalyzed Cycloisomerization of Alkynediols: Asymmetric Total Synthesis of Farnesiferol C
Yue-Qing Gu, Peng-Peng Zhang, Jun-Kai Fu, Song Liu, Yu Lan,* Jian-Xian Gong,* and Zhen Yang*
Adv. Synth. Catal. 2016, 358, 1392-1397
A highly regio- and stereoselective method to construct a broad range of 7-oxabicyclo[2.2.1]heptanes, which proceeds through a sequential reaction involving gold(I)-catalyzed cycloisomerization of alkynediols and sequential semi-pinacol-type 1,2-alkyl migration, was developed. The developed chemistry was applied to the asymmetric total synthesis of the natural product farnesiferol C.