Publications
175.Exo-Selective Diels–Alder Reactions
Yuan-He Li,* Jia-Hua Chen, and Zhen Yang*
Chem. Eur. J. 2024, 30(17), e202304371
The Diels–Alder reaction stands as one of the most pivotal transformations in organic chemistry. Its efficiency, marked by the formation of two carbon-carbon bonds and up to four new stereocenters in a single step, underscores its versatility and indispensability in synthesizing natural products and pharmaceuticals. The most significant stereoselectivity feature is the “endo rule”. While this rule underpins the predictability of the stereochemical outcomes, it also underscores the challenges in achieving the opposite diastereoselectivity, making the exo-Diels–Alder reactions often considered outliers. This review delves into recent examples of exo-Diels–Alder reactions, shedding light on the factors inverting the intrinsic tendency. We explore the roles of steric, electrostatic, and orbital interactions, as well as thermodynamic equilibriums in influencing exo/endo selectivity. Furthermore, we illustrate strategies to manipulate these factors, employing approaches such as bulky substituents, s-cis conformations, transient structural constraints, and innovative control physics. Through these analyses, our aim is to provide a comprehensive understanding of how to predict and design exo-Diels–Alder reactions, paving the way for new diastereoselective catalyst systems and expanding the chemical scope of Diels–Alder reactions.
174.Regioselective Syntheses of 1,4- and 1,6-Dicarbonyl Compounds via Photoredox-Based Oxidative Heterocoupling of Enolsilanes with Oxygen as an Oxidant
Xinyu Tan, Zhilin Song, Xinting Liang, Zhenbao Wang, Hongyi Yuan, Zhongchao Zhang,*and Zhen Yang*
Org. Lett. 2024, 26(26), 5403−5408
A photoredox-based oxidative heterocoupling of enolsilanes to the corresponding 1,4- and 1,6-dicarbonyl compounds was developed by using Mes-Acr+BF4– as the photocatalyst, and oxygen was used as the oxidant. This newly developed chemistry adheres to the principles of atom economy, step economy, and redox economy, making it a concise and efficient method.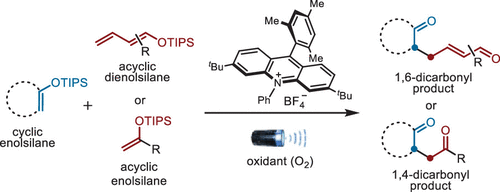
173.Total Synthesis of (+)−Haperforin G
Zhenyu Zhang, Wei Zhang, Jun−Chen Tang, Jin−Teng Che, Zhongchao Zhang, Jia−Hua Chen,*and Zhen Yang*
J. Org. Chem. 2023, 88(15), 10539−10554
(+)-Haperforin G was synthesizedin 20 steps from commerciallyavailable starting materials. A Co-catalyzed intramolecular Pauson-Khandreaction was used for stereoselective construction of cyclopentanonebearing an all-carbon quaternary stereogenic center at the bridge-headposition. Light-initiated photocatalysis was used for convergent andasymmetric cross-coupling of the unstabilized C(sp(3)) radicalwith an enone. The developed chemistry paves the way to the synthesisof structurally diverse analogs of haperforin G (6).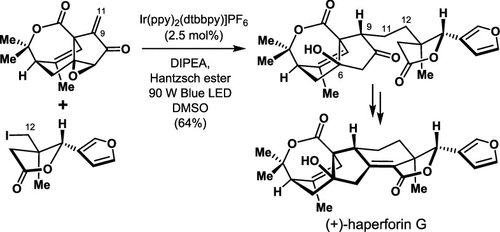
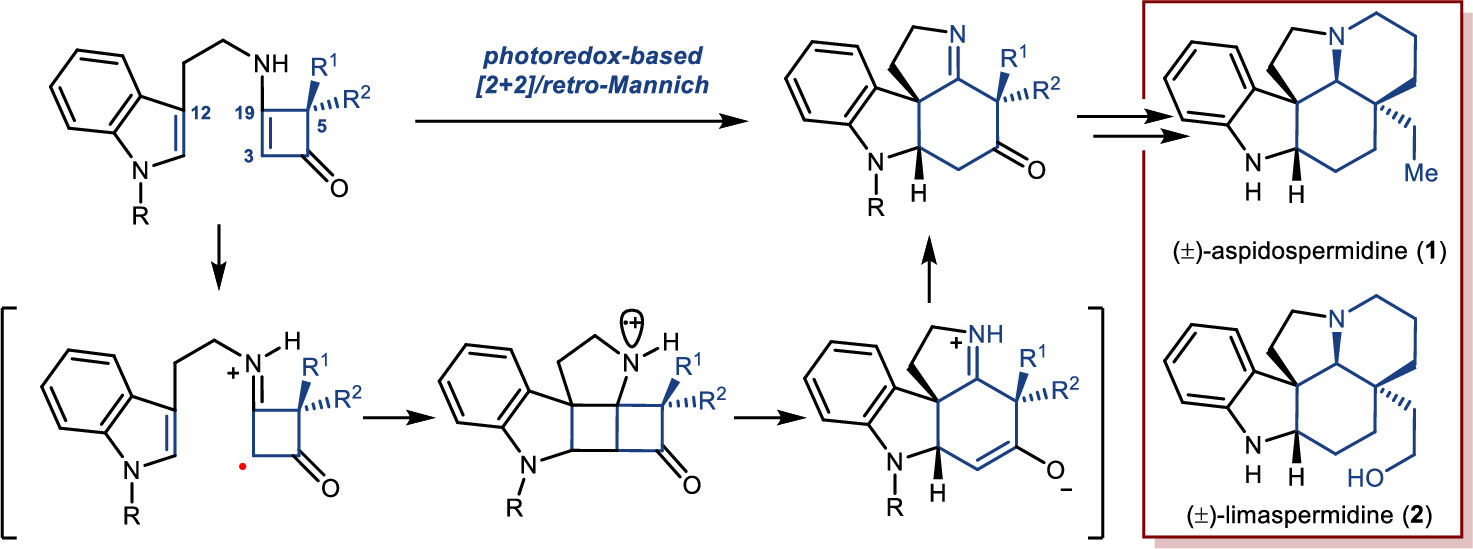
172.Development of a Strategy for the Total Synthesis of Aspidosperma Alkaloids via the Cyclobutenone-Based PET-Initiated Cationic Radical-Driven [2+2]/Retro-Mannich Reaction
Jianyu Long, Rudong Liu, Xinpeng Mu, Zhilin Song, Zhongchao Zhang,* and Zhen Yang*
Org. Lett. 2024, 26(15), 2960–2964
A novel strategy for the synthesis of Aspidosperma alkaloids has been achieved via a photoredox-initiated [2+2]/retro-Mannich reaction of tryptamine-substituted enaminones as a key step. The developed chemistry has been applied to the construction of the core tetracycle of Aspidosperma alkaloids (±)-aspidospermidine and (±)-limaspermidine.
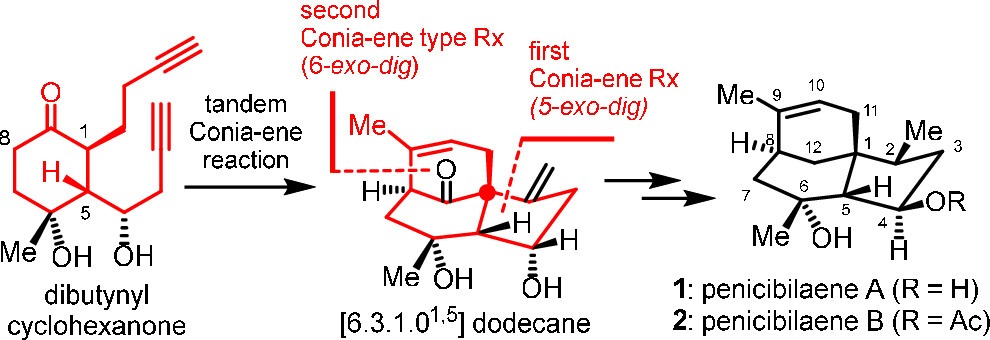
171.Total Synthesis of Penicibilaenes Enabled by a Tandem Double Conia-ene Type Reaction
Zheyuan Wang, Zhilin Song, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2024, 146(7), 4363–4368
The total syntheses of penicibilaenes A and B are described. The key step is the tBuOK/DMSO-mediated tandem 5-exo–dig Conia-ene type reaction and 6-exo–dig Conia-ene type reaction to install the tricyclic [6.3.1.01,5] dodecane core of penicibilaenes from dibutynyl cyclohexanone in a single step, together with a sequence of copper-mediated conjugate addition and Crabtree’s hydrogenation to forge the stereogenic centers at C5 and C2, respectively.