106.Effective Chirality Transfer in [3+2] Reaction between Allenyl-Rhodium and Enal: Mechanistic Study Based on DFT Calculations
Xiaotian Qi, Song Liu, Tao Zhang, Rong Long, Jun Huang, Jianxian Gong*, Zhen Yang*, and Yu Lan*
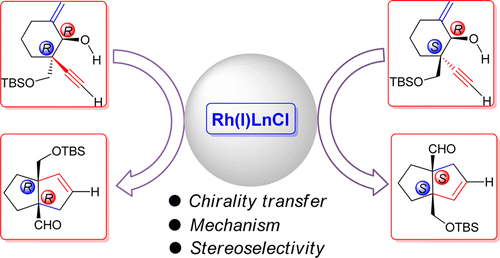
Theoretical calculation was performed to study the chirality transfer in a newly reported intramolecular [3+2] cycloaddition of enal and alleno rhodium species, generated in situ from an enynol precursor. [3.3.0] bicyclic system which contains two bridgehead quaternary carbons that can be achieved, the chirality of which are controlled by those of the starting material, and the product stereoselectivity is only determined by the α-position of the acetylene moiety. Density functional theory calculations predicted that only the cis [3.3.0] bicyclic product could be generated, regardless of either erythro or threo substrate, which was also confirmed by experimental observations.