121. Enantioselective Total Synthesis of (−)-Pavidolide B
Peng-Peng Zhang, Zhi-Ming Yan, Yuan-He Li, Jian-Xian Gong*, and Zhen Yang*
J. Am. Chem. Soc., 2017, 139 , 13989–13992
• Second Most Read Articles in JACS 2017/10.
• Highlighted in Synfacts, 2017, 13, 1233.
• Highlighted in Org. Chem. Highlights 2017, April 2.
• Highlighted in ChemistryWorld, 2017, Nov, 1.
• Highlighted in Chin. J. Org. Chem. 2018, 38, 282.
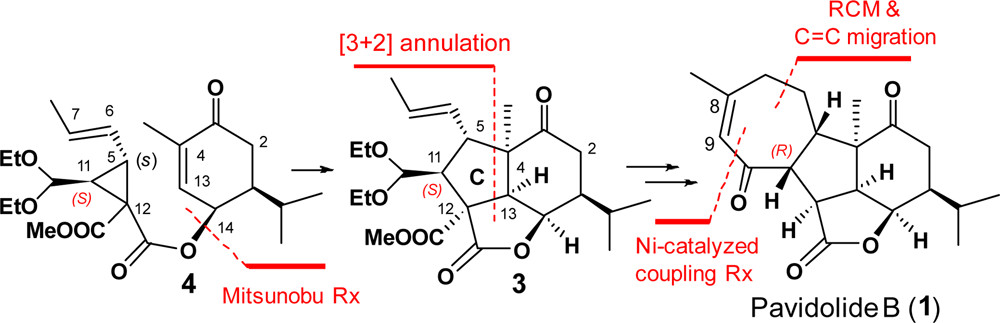
The enantioselective synthesis of (−)-pavidolide B (1) was achieved in a linear sequence of 10 steps. The key steps are (a) an enantioselective organocatalytic cyclopropanation; (b) a radical-based cascade annulation for the regio- and diastereo-selective synthesis of the highly functionalized lactone 3 bearing the characteristic tricyclic core and seven contiguous stereocenters; (c) a sequential ring-closing metathesis reaction and a RhCl3-catalyzed double bond isomerization to form the seven-membered D ring of (−)-pavidolide B.