143.Asymmetric Total Synthesis of (+)-Waihoensene
Yongzheng Qu, Zheyuan Wang, Zhongchao Zhang, Wendou Zhang, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(14), 6511-6515
• Highlighted in ChemistryViews, 07 Apr 2020
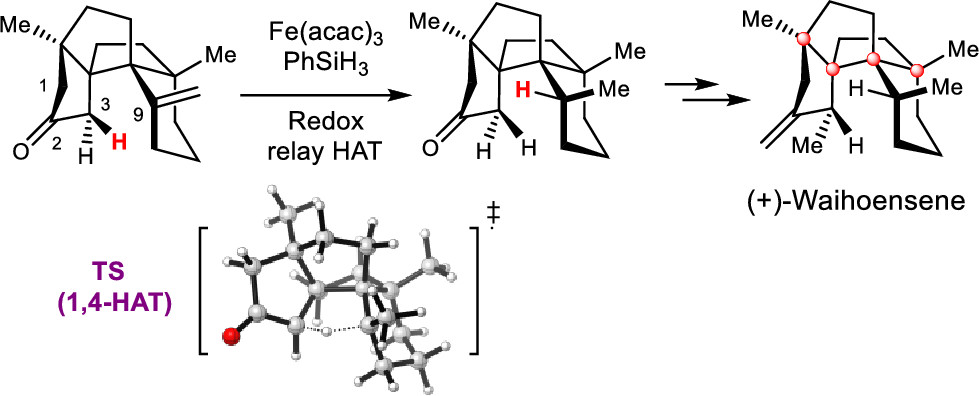
The asymmetric total synthesis of (+)-waihoensene, which has a cis-fused [6,5,5,5] tetracyclic core bearing an angular triquinane, a cis-fused six-membered ring, and four contiguous quaternary carbon atoms, was achieved through a sequence of chemical reactions in a stereochemically well-defined manner. The total synthesis features the following: (1) Cu-catalyzed asymmetric conjugated 1,4-addition; (2) diastereoselective Conia-ene type reaction; (3) diastereoselective intramolecular Pauson–Khand reaction; (4) Ni-catalyzed diastereoselective conjugated 1,4-addition; and (5) radical-initiated intramolecular hydrogen atom transfer (HAT). Control experiments and density functional theory calculations support the proposed HAT process.