155.Stereoselective Synthesis of trans-Decalin-Based Spirocarbocycles via Photocyclization of 1,2-Diketones
Sijia Chen, Zhongchao Zhang, Chongguo Jiang, Chunbo Zhao, Haojie Luo, Jun Huang*, and Zhen Yang*
ACS Omega 2021, 6(29), 18848–18859
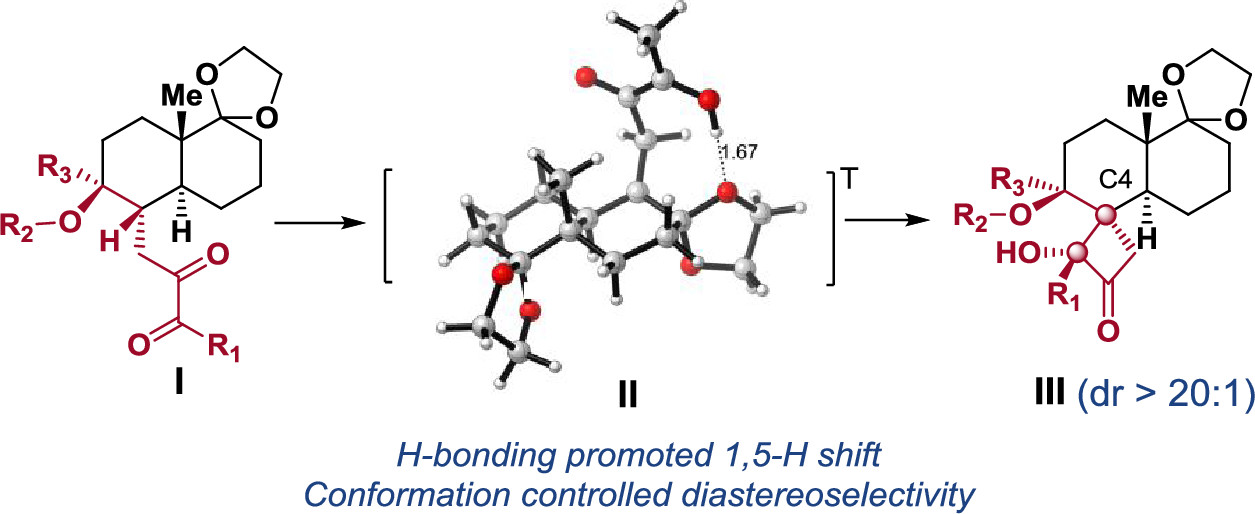
Diastereoselective synthesis of the trans-decalin-based α-hydroxyl butanone spirocarbocycles bearing all-carbon quaternary stereogenic centers has been achieved via Norrish–Yang photocyclization of trans-decalin-substituted-2,3-butanediones using daylight. Density functional theory (DFT) calculations suggest that this diastereoselective reaction is affected by both substrate conformation and intramolecular hydrogen bonds. The developed chemistry could be applied to synthesizing the derivatives of the trans-decalin-based biologically important natural products.