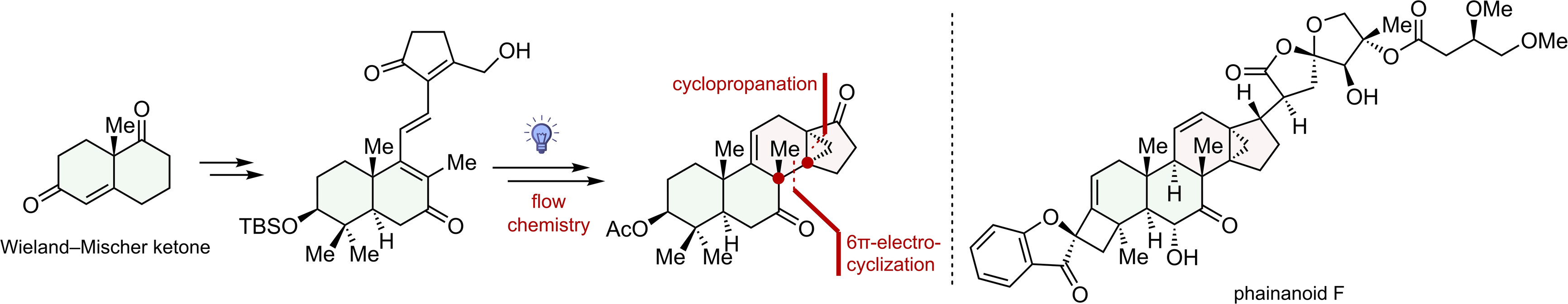
170.Synthesis towards Phainanoid F: Photo-induced 6π- Electrocyclization for Constructing Contiguous All-Carbon Quaternary Centers
Hao-Yuan Liu, Zhen-Yu Zhang, Yi-Ke Zhou, Jia-Hua Chen, Zhen Yang,* and Yuan-He Li*
Chem Asian J.2023,18, e20230062
In this paper, we report an efficient strategy for synthesizing the DEFGH rings of phainanoid F. The key to the construction of the 13,30-cyclodammarane skeleton of the molecule was a photo-induced 6π-electrocyclization and a homoallylic elimination. Notably, this is a rare example of using electrocyclization reaction to simultaneously construct two vicinal quaternary carbons in total synthesis. The strategy outlined here forms the basis of our total synthesis of Phainanoid F, and it could also serve as a generally applicable approach for synthesizing other natural products containing similar 13,30-cyclodammarane skeletons.