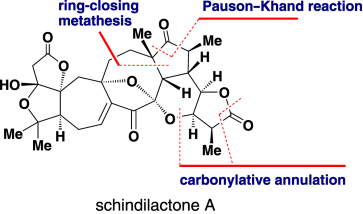
59.Diastereoselective Total Synthesis of (±)-Schindilactone A
Q. Xiao, W. W. Ren, Z. X. Chen, T. W. Sun, Y. Li, Q. D. Ye, J. X. Gong, F. K. Meng, L. You, Y. F. Liu, M. Z. Zhao, L. M. Xu, Z. H. Shan, Y. Shi,Y. F. Tang*, J. H. Chen*, Z. Yang*
Angew. Chem. Int. Ed. 2011, 50, 7373.
All together: A concise strategy for the first diastereoselective total synthesis of (±)-schindilactone A is reported. The synthesis features a ring-closing metathesis, a thiourea/cobalt-catalyzed Pauson–Khand reaction, and a thiourea/palladium-catalyzed carbonylative annulation reaction. The chemistry can be applied to the synthesis of structures related to schindilactone A.