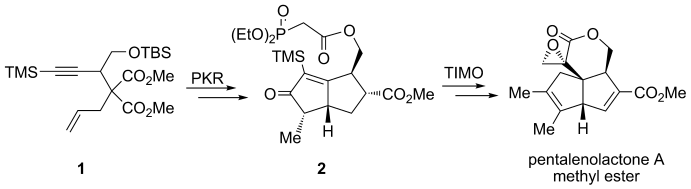
61.Total Synthesis of (±)-Pentalenolactone A Methyl Ester
Q. Liu, G. Z. Yue, N. Wu, G. Lin, Y. Z . Li, J. M. Quan, C. C. Li*, G. X. Wang*, Z. Yang*
Angew. Chem. Int. Ed. 2012, 51, 48, 12072
• Top 10 Most Accessed Article in 10/2012.
• Highlighted in Chin. J. Org. Chem., January, 2013.
• Highlighted in Chemistry World, November, 2012.
Ringing it up: The methyl ester of pentalenolactone A has been obtained through the stereoselective synthesis of a cyclopentenone by a combination of the Co-mediated Pauson–Khand reaction (PKR) of enyne 1, and the construction of a quaternary-carbon-based strained α-methylidene-δ-pentyrolactone core through a trimethylsilyl (TMS) mediated, telescoped intramolecular Michael olefination (TIMO) reaction of keto-phosphonate 2.