63.Enantioselective and Collective Syntheses of Xanthanolides Involving a Controllable Dyotropic Rearrangement of cis-b -Lactones
W. W. Ren, Y. C. Bian, Z. Y. Zhang, H. Shang, P. T. Zhang, Y. J. Chen, Z. Yang*, T. P. Luo*, and Y. F. Tang*
Angew. Chem. Int. Ed. 2012, 51, 6984
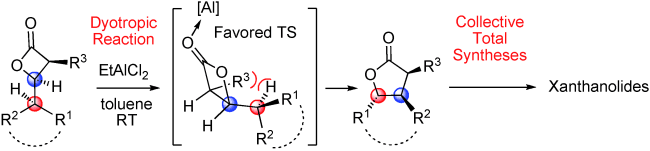
Let’s swap: A scalable, atom-economic, enantio-, and diastereoselective synthetic route to trisubstituted γ-butyrolactones based on a Wagner–Meerwein-type dyotropic rearrangement of cis-β-lactones is described (see scheme). This methodology was applied in efficient and protecting-group-free formal syntheses and total syntheses of various xanthanolide natural products.