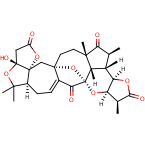
65.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 2: Construction of the Fully Functionalized CDEFGH Ring System
Yong Li, Zhi-Xing Chen, Dr. Qing Xiao, Qin-Da Ye, Tian-Wen Sun, Fan-Ke Meng, Dr. Wei-Wu Ren, Lin You, Ling-Min Xu, Yue-Fan Wang, Dr. Jia-Hua Chen,*, Dr. Zhen Yang,*
The successful synthesis of the highly complex model compound (2) of the CEFGH ring system of schindilactone A (1) is described. Several synthetic methodologies were developed and applied to achieve this goal, including ring-closing metathesis (RCM) and Co–thiourea-catalyzed Pauson–Khand reactions. Furthermore, two independent approaches were developed for the construction of the GH ring of model compound 2, the key steps of which included Pd–thiourea-catalyzed carbonylative annulation, methylation, and sequential RCM/oxa-Michael-addition reactions. The chemistry developed herein has provided a greater understanding of the synthesis of schindilactone A (1) and its analogous compounds of the same family.