75.Asymmetric Total Synthesis of Caribenol A via an Intramolecular Diels−Alder Reaction
Jing-Chun Han, Lian-Zhu Liu, Yuan-Yuan Chang, Guo-Zong Yue, Jie Guo, Li-Yan Zhou, Chuang-Chuang Li*, and Zhen Yang*
-
• Highlighted in Synfacts, 2013, 9, 809.
• Highlighted in Org. Chem. Highlights, December, 2013.
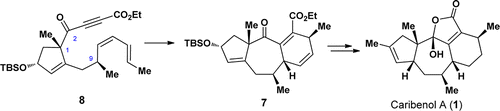
A total synthesis of the caribenol A (1), a novel natural product with an intriguing tetracyclic framework, has been achieved. The synthesis features an intramolecular Diels–Alder (IMDA) reaction for the facile construction of the tricyclic [5–7–6] skeleton of caribenol A (1) and a biomimetic oxidation reaction for the formation of the 2-hydroxyfuran-2(5H)-one motif of caribenol A (1) as key steps. This synthetic approach also reveals that the sp2 carbon at C(2) in substrate 8 is a critical factor for the formation of the tricyclic [5–7–6] skeleton in 7.