99.Towards a general diastereoselective route to oxabicyclo[3.2.1]octanes via a gold-catalysed cascade reaction
Junkai Fu, Yueqing Gu, Hao Yuan, Tuoping Luo, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
Nat. Commun., 2015, 6:8617. DOI: 10.1038/ncomms9617 (2015)
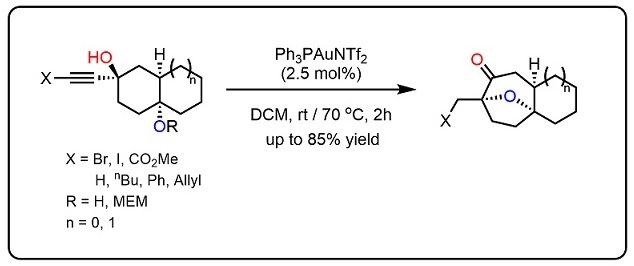
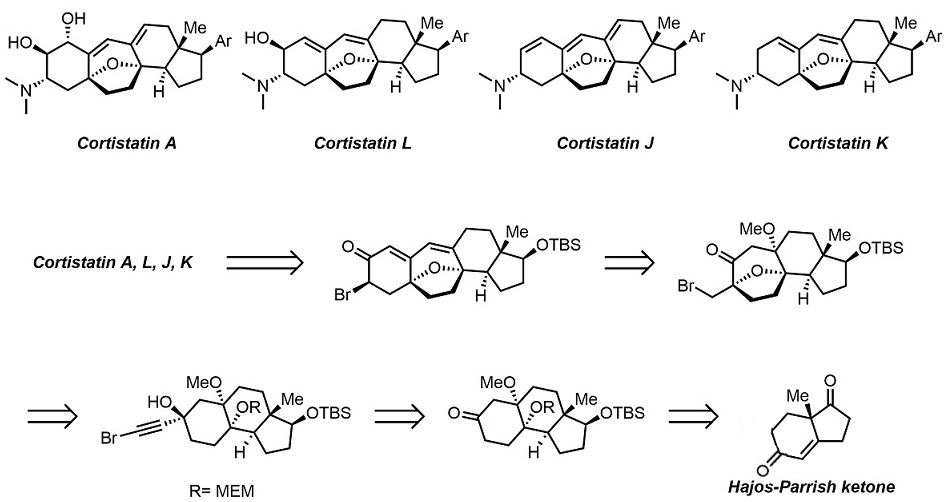
The development of an efficient diastereoselective synthesis of the oxabicyclo[3.2.1]octane ring system bearing two oxygenated quaternary chiral centres represents a significant challenge. This motif can be found in a wide range of natural products with significant biological activities. Here we report the synthesis of such kind of scaffold using a cyclohexane-trans-1,4-diol with an alkyne side chain in the presence of Au(I) catalyst. This is a domino process in which two C–H, two C–O and one C–C bond is assembled through a sequence of cyclization/semi-pinacol rearrangements. This strategy has been successfully applied to the asymmetric formal total synthesis of (+)-cortistatins.