102.Isolation and Asymmetric Total Synthesis of Perforanoid A
Chao Lv , Xiaohui Yan , Qian Tu, Yingtong Di, Chunmao Yuan, Xin Fang, Yaacove Ben-David, Lei Xia, Jianxian Gong, Yuemao Shen,* Zhen Yang,* and Xiaojiang Hao*
Angew. Chem. Int. Ed. 2016, 55, 7539
• Highlighted in Org. Chem. Highlights 2017, March 6.
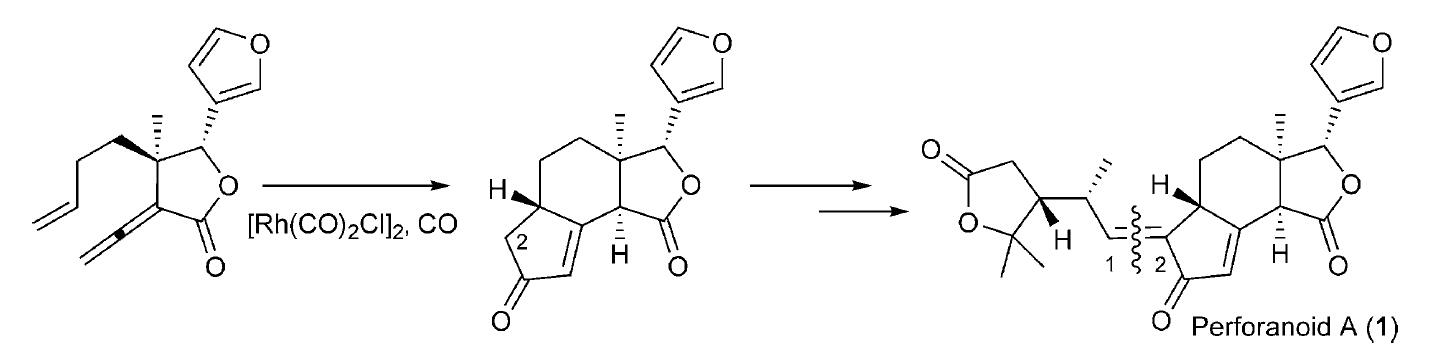
A novel limonoid, perforanoid A, was isolated, and an asymmetric total synthesis was achieved in 10 steps. The key steps are chiral tertiary aminonaphthol mediated enantioselective alkenylation of an aldehyde to an allylic alcohol, Pd-catalyzed coupling of the allylic alcohol with vinyl ether to form the γ-lactone ring, and cyclopentenone ring formation through a Rh-catalyzed Pauson–Khand reaction. Preliminary studies show that perforanoid A is cytotoxic towards HEL, K562, and CB3 tumor cell lines.