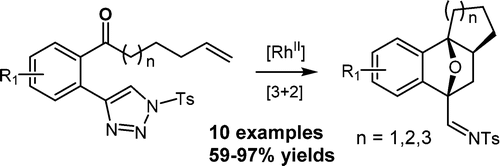
108.Stereoselective Synthesis of Oxabicyclo[2.2.1]heptenes via a Tandem Dirhodium(II)-Catalyzed Triazole Denitrogenation and [3 + 2] Cycloaddition
Hao Yuan, Jianxian Gong*, and Zhen Yang*
Org. Lett., 2016, 18 (21), 5500–5503
A novel synthetic strategy for the diastereoselective synthesis of structurally diverse oxabicyclo[2.2.1]heptenes has been developed, featuring a tandem reaction combining a Rh-catalyzed triazole denitrogenation and a novel type of [3 + 2] cycloaddition reaction. This tandem reaction was thought to proceed via a five-membered oxonium ylide intermediate, which was formed by the intramolecular nucleophilic attack of the carbonyl group on the α-imino metallocarbene followed by an inter- or intramolecular [3 + 2] dipolar cycloaddition with a range of alkynes and alkenes.