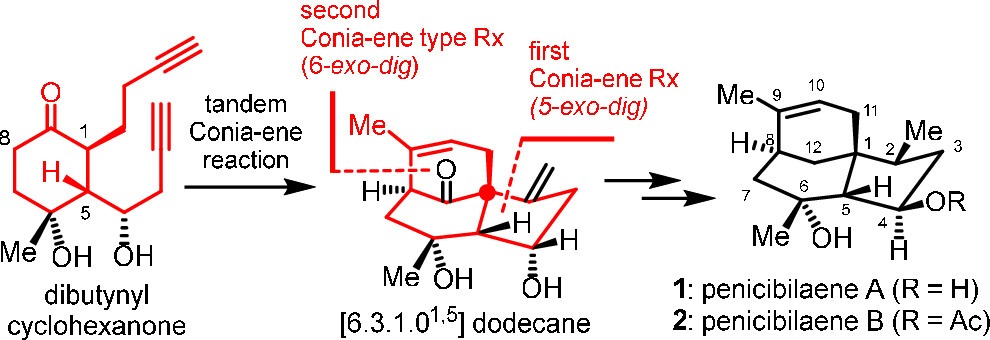
171.Total Synthesis of Penicibilaenes Enabled by a Tandem Double Conia-ene Type Reaction
Zheyuan Wang, Zhilin Song, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2024, 146(7), 4363–4368
The total syntheses of penicibilaenes A and B are described. The key step is the tBuOK/DMSO-mediated tandem 5-exo–dig Conia-ene type reaction and 6-exo–dig Conia-ene type reaction to install the tricyclic [6.3.1.01,5] dodecane core of penicibilaenes from dibutynyl cyclohexanone in a single step, together with a sequence of copper-mediated conjugate addition and Crabtree’s hydrogenation to forge the stereogenic centers at C5 and C2, respectively.