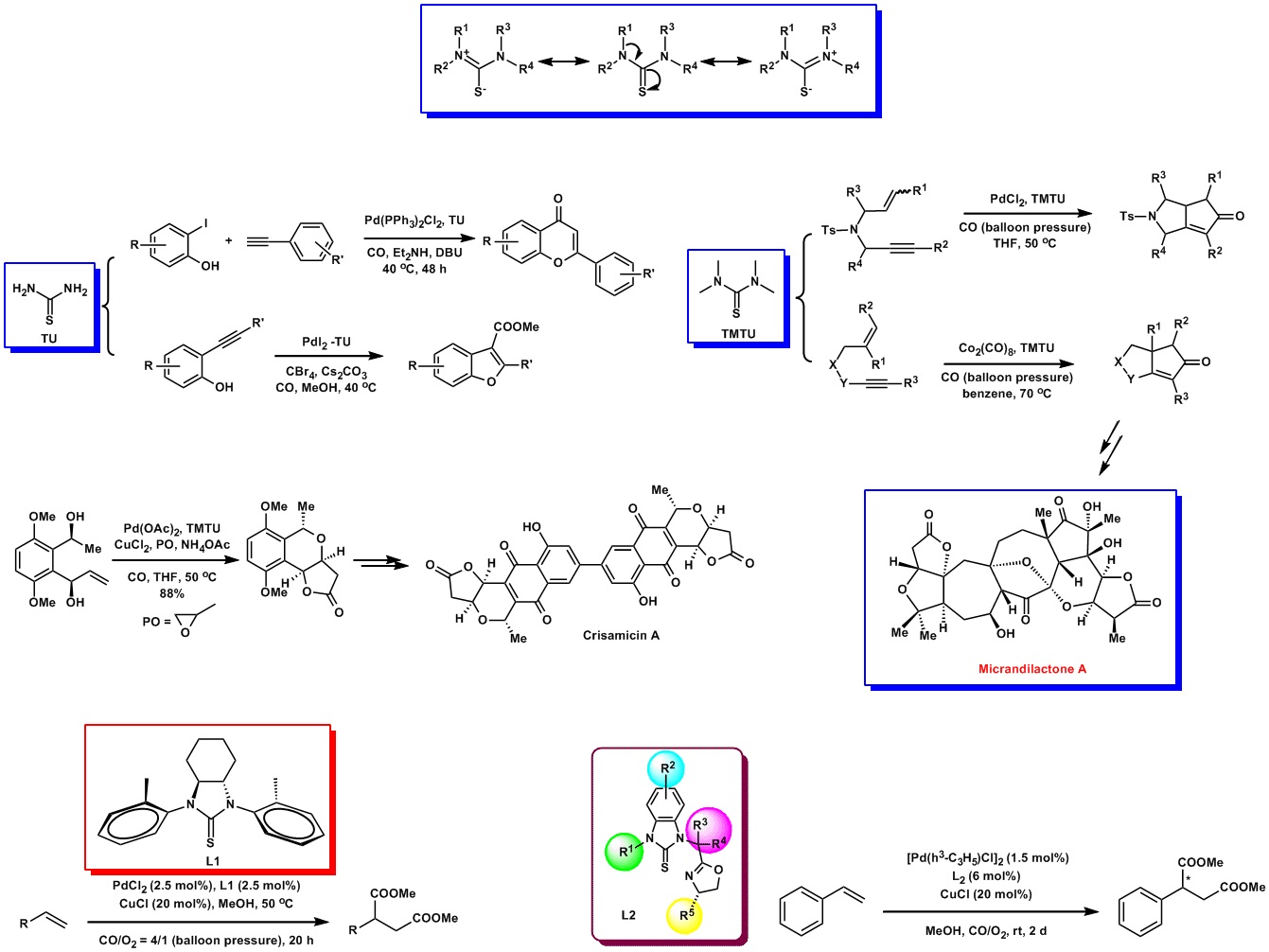
New Synthetic Methodology Program
The Pd-catalyzed cross-coupling reaction is a method for C-C bond formation. In this context, the design of novel ligands and transition metal catalysts that can effect desired transformations, with both high efficiency and selectivity, has been a fertile area of research. Phosphine-based ligands have been playing an important role in the Pd-catalyzed cross-coupling reactions. However, these ligands usually need to be handled in an inert atmosphere or in dry conditions, sometimes suffer from significant P-C bond degradation at elevated temperatures, which leads to palladium aggregation and do ham to the catalytic activity.
Recently, phosphine-free ligands, such as N-heterocyclic carbenes(NHC), and sulfur-containing ligands have been applied in some metal-catalyzed synthetic transformations, which open up new opportunities in catalysis.
Thioureas are air and moisture stable compounds and are renowned for their great tenability by varying substituents on nitrogen, so that they can coordinate to metal centers in either the neutral state or as mono-anion or dianion forms. Therefore, their physical and chemical properties can be changed by modifying their nitrogen substituents.
We recently synthesized two-type of thioureas L1 and L2, which were effectively applied to the Pd-catalyzed several types of carbonylative reactions and cross-coupling reactions. We are currently working on the investigation of the scope and limitations of our ligands in a broad spectrum of reactions.