Rhodium(III)-Catalyzed Indazole Synthesis by C−H Bond Functionalization and Cyclative Capture
Transition metal catalyzed C-H functionalization has emerged as a powerful tool to build molecular complexity from widely available starting materials. It has been widely used in the synthesis of natural products and drugs. There are many reviews on heterocycle synthesis through C-H functionalization, but indazole synthesis by formal [4+1] annulation with aldehydes have not been reported.
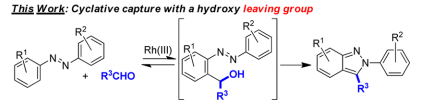
Recently, Ellman et. al. reported Rhodium(III)-catalyzed indazole synthesis by C−H bond functionalization and cyclative capture. (J.Am.Chem.Soc., 2013,135,7122 DOI:10.1021/ja402761p) This is an efficient, one-step, and highly functional group-compatible synthetic method.
Here the hydroxy prefers to be a leaving group rather than be a nucleophile.
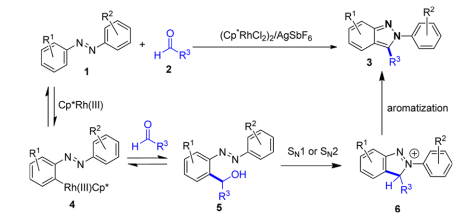
Plausible mechanism:
The azo functional group would direct ortho C−H bond activation, followed by the reversible addition to aldehyde 2 to provide alcohol 5. Cyclative capture by an intramolecular nucleophilic substitution to give 6 followed by rapid aromatization should provide the desired 2H-indazole 3.
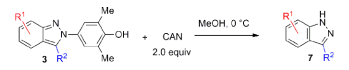
N-aryl-2H-indazoles can be oxidized with ceric ammonium nitrate (CAN) in methanol, affording indazoles without N-substitution. Chloro, methyl, nitro, and methoxy groups are all well tolerated.
The author also pointed that the 2-aryl-2H-indazole products represented a new class of readily prepared fluorophores.