RESEARCH
Current Research Focus
1. To understand IP3 and IP3R-mediated calcium signals and signaling pathways
2. To identify novel substrates for protein kinase C and protein kinase D
3. To investigate the physiological function of IP3R, PKC, and PKD in cardiovascular system and metabolism using gene knockout mouse models
4.To mimic the pathogenesis and progression of human cardiovascular diseases using human patient induced pluripotent stem (iPS) cells
5. To develop novel technology, cell and animal models for small molecule screening
Technical Expertise
1. Electrophysiology: whole cell recording, single channel recording, intracellular recording
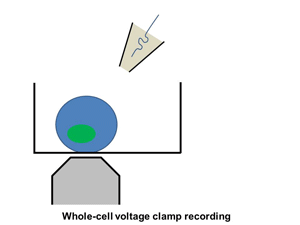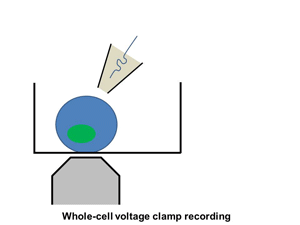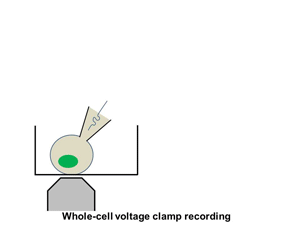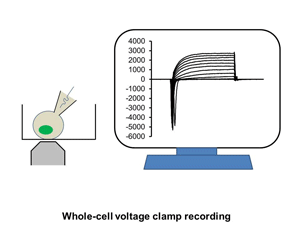
Ion channels such as Na+ channel, K+ channel, Ca2+ channel, and IP3R, are pore-forming membrane proteins localized in the plasma membrane or the membrane of intracellular organelles, which contribute to establish a resting membrane potential, shape action potential and other electrical signals in excitable cells (i.e. neurons, cardiomyocytes, and skeletal muscles), control the flow of ions across secretory and epithelial cells, and regulate cell volume. It has been demonstrated that a number of genetic disorders of ion channels and their modifiers disrupt normal functioning of ion channels and have disastrous consequences for the organism. We are utilizing patch clamp and voltage clamp techniques to study electrophysiological changes of cardiac cells during differentiation, maturation, and disease progression. We also use these techniques to examine the effects of small molecules on the properties of diverse ion channels. The video shows approaching of an electrode to a cell, formation of the whole-cell mode, and an example of the currents recorded under the voltage-clamp mode.
2. Confocal calcium imaging
Ca2+ is a ubiquitous and versatile signaling molecule that regulates many cellular processes including muscle contraction, neuronal excitability, hormone secretion, cell proliferation, and cell death. There are two major ways to increase cytosol Ca2+ concentration: Ca2+ entry via Ca2+ channels on plasma membrane and Ca2+ release via RyR or IP3R from intracellular Ca2+ store. Ca2+ signals are highly temporally (from ms to hours) and spatially (from um to cm) organized inside the cell, which allows Ca2+-mediated signaling molecules to be recruited and specific actions to be initiated. We are using laser confocal microscope and various Ca2+ dyes to study how intracellular Ca2+ signals are generated and how they play their cellular functions. The video shows spontaneous calcium transients and cell beatings in cardiomyocytes differentiated from human iPS cells.
3. Cardiovascular physiology (Echocardiographic and Doppler imaging, ECG, Blood pressure, Hemodynamics)
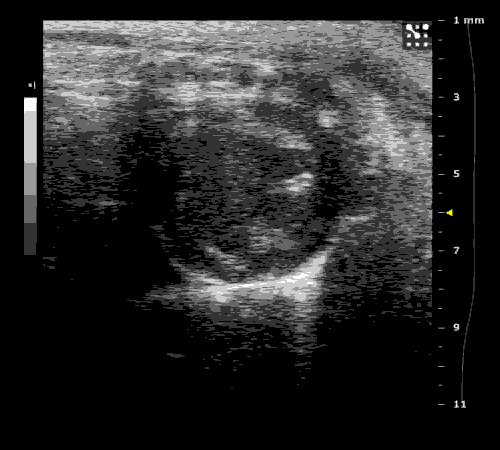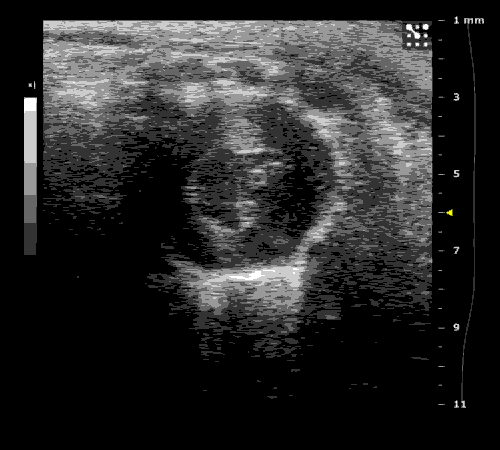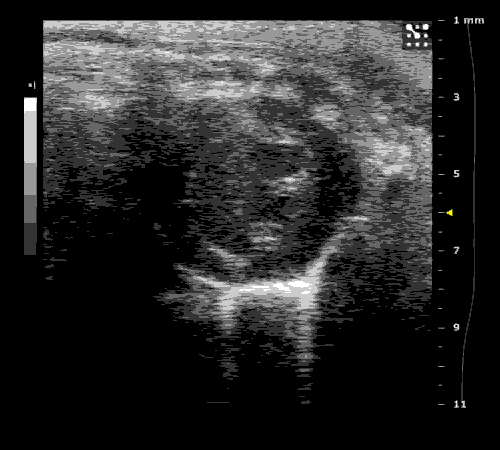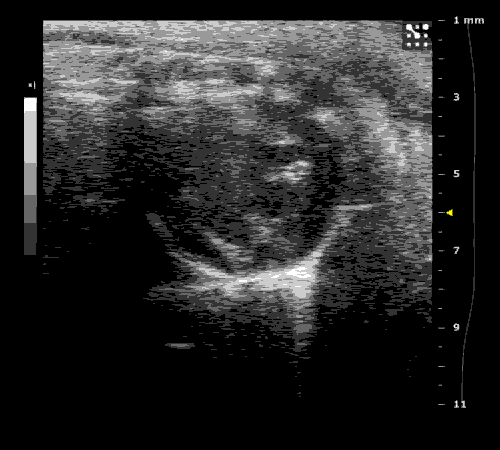
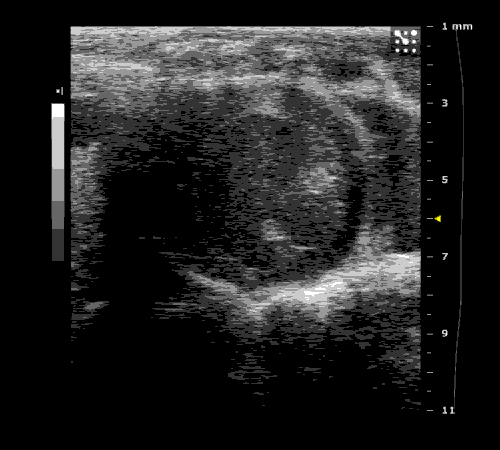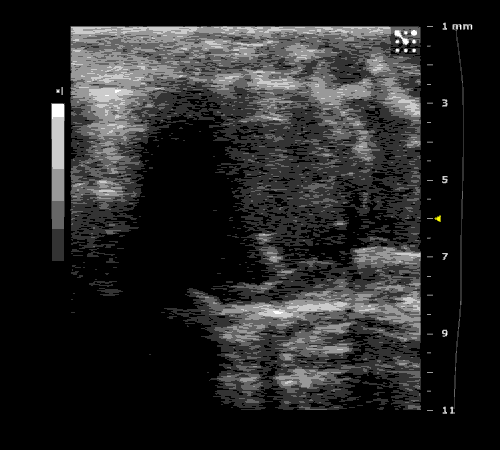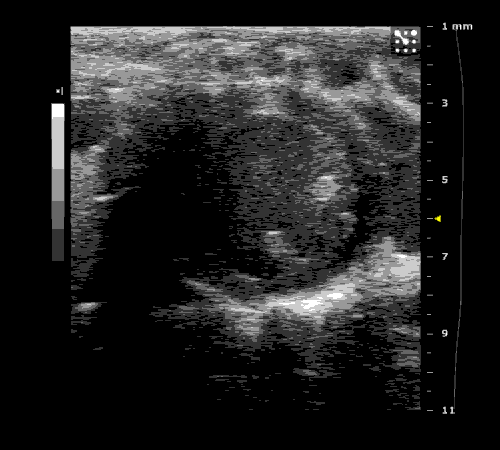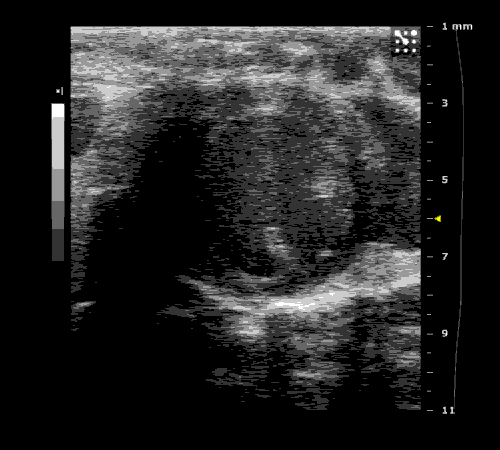
We applied multiple techniques to study cardiovascular physiology of transgenic animals and surgical models of chronic myocardial infarction, heart failure and cardiac hypertrophy. Non-invasive echocardiographic and Doppler imaging is used to evaluate cardiac mechanisms and function. Telemetry recording is applied to measure blood pressure and ECG in conscious animals. We also use invasive hemodynamic measurements to assess cardiac contractility, cardiac output, ejection fraction, LV and aortic blood pressure. The video clips show short axis views of ultrasound on a normal (left) heart and a failing (right) heart.