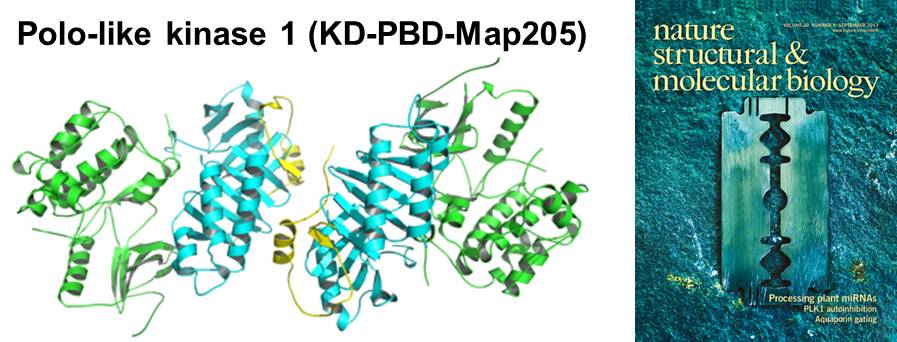
Structural basis for the inhibition of Polo-like kinase 1- Highlighted Research by Nature China
Polo-like kinase 1 (PLK1) is a master regulator of mitosis and is considered a potential drug target for cancer therapy. PLK1 is characterized by an N-terminal kinase domain (KD) and a C-terminal Polo-box domain (PBD). The KD and PBD are mutually inhibited, but the molecular mechanisms of the autoinhibition remain unclear. Here we report the 2.3-Å crystal structure of the complex of the Danio rerio KD and PBD together with a PBD-binding motif of Drosophila melanogaster microtubule-associated protein 205 (Map205PBM). The structure reveals that the PBD binds and rigidifies the hinge region of the KD in a distinct conformation from that of the phosphopeptide-bound PBD. This structure provides a framework for understanding the autoinhibitory mechanisms of PLK1 and also sheds light on the activation mechanisms of PLK1 by phosphorylation or phosphopeptide binding.
Xu, J., Shen, C., Wang, T. et al. Structural basis for the inhibition of Polo-like kinase 1. Nat Struct Mol Biol 20, 1047–1053 (2013). https://doi.org/10.1038/nsmb.2623
(This work has been highlighted in cover and also by Nature China, 2 October 2013 | doi:10.1038/nchina.2013.92)